Abstract
Purpose
Intelligent biosynthetic nanobiomaterials (IBNs) were constructed as recombinant diblock copolymers, notated as K8-ELP(1–60), containing a cationic oligolysine (VGK8G) and a thermosensitive elastin-like polypeptide (ELP) block with 60 repetitive pentapeptide units [(VPGXG)60; X is Val, Ala and Gly in a 5:2:3 ratio].
Methods
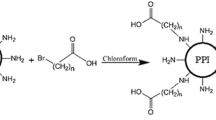
K8-ELP(1–60) was synthesized by recursive directional ligation for DNA oligomerization. Purity and molecular weight of K8-ELP(1–60) were confirmed by SDS-PAGE and mass spectrometry. DNA polyplexes were prepared from K8-ELP(1–60) and pGL3-Control (pGL3–C) plasmid DNA (pDNA) and stability was evaluated by gel retardation, DLS, and DNA displacement with heparin. Thermal transition profiles were studied by measuring the turbidity change at 350 nm and the polyplexes were used to transfect MCF-7 cells with a concomitant cytotoxicity assay.
Results
SDS-PAGE and MALDI-TOF studies showed highly pure copolymers at the desired molecular weight. K8-ELP(1-60) condensed pDNA at a cation to anion (N/P) ratio above 0.25 with a tight distribution of particle size ranging from 115.5–32.4 nm with increasing N/P ratio. Thermal transition temperatures of K8-ELP(1-60)/pDNA and K8-ELP(1-60) alone were 44.9 and 71.5°C, respectively. K8-ELP(1-60)/pDNA complexes successfully transduced MCF-7 cells with qualitative expression of enhanced green fluorescent protein (EGFP) and minimal cytotoxicity compared to branched poly(ethyleneimine) controls.
Conclusions
K8-ELP(1-60) was successfully designed and purified through recombinant means with efficient and stable condensation of pDNA at N/P ratios > 0.25 and polyplex particle size < 115 nm. MCF-7 cells successfully expressed EGFP with minimal cytotoxicity compared to positive controls; moreover, polyplexes retained sharp, thermotransitive kinetics within a narrow Tt range at clinically relevant hyperthermic temperatures, where the decrease of Tt was due to the increased hydrophobicity upon charge neutralization.








Similar content being viewed by others
Abbreviations
- ELP:
-
elastin-like polypeptide
- ELP(1–60):
-
ELP with 60 repetitive pentapeptide units [(VPGXG)60, X is Val, Ala and Gly in a 5:2:3 ratio]
- IBN:
-
intelligent biosynthetic nanobiomaterial
- ITC:
-
inverse transition cycling
- K8 :
-
oligolysine (VGK8G)
- RDL:
-
recursive directional ligation
- Tt (°C):
-
phase transition temperature
References
P. Factor. Gene therapy for acute diseases. Mol. Ther. 4:515–524 (2001).
S. Yla-Herttuala, and K. Alitalo. Gene transfer as a tool to induce therapeutic vascular growth. Nat. Med. 9:694–701 (2003).
J. T. Santoso, D. C. Tang, S. B. Lane, J. Hung, D. J. Reed, C. Y. Muller, D. P. Carbone, J. A. Lucci 3rd, D. S. Miller, and J. M. Mathis. Adenovirus-based p53 gene therapy in ovarian cancer. Gynecol. Oncol. 59:171–178 (1995).
F. Siddiqui, E. J. Ehrhart, B. Charles, L. Chubb, C. Y. Li, X. Zhang, S. M. Larue, P. R. Avery, M. W. Dewhirst, and R. L. Ullrich. Anti-angiogenic effects of interleukin-12 delivered by a novel hyperthermia induced gene construct. Int. J. Hyperthermia 22:587–606 (2006).
J. Zabner, A. J. Fasbender, T. Moninger, K. A. Poellinger, and M. J. Welsh. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 270:18997–19007 (1995).
P. C. Bell, M. Bergsma, I. P. Dolbnya, W. Bras, M. C. Stuart, A. E. Rowan, M. C. Feiters, and J. B. Engberts. Transfection mediated by gemini surfactants: engineered escape from the endosomal compartment. J. Am. Chem. Soc. 125:1551–1558 (2003).
J. Y. Legendre, and F. C. Szoka Jr. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm. Res. 9:1235–1242 (1992).
X. Gao, and L. Huang. Cationic liposome-mediated gene transfer. Gene Ther. 2:710–722 (1995).
O. Boussif, F. Lezoualc’h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A. 92:7297–7301 (1995).
Z. Megeed, M. Haider, D. Li, B. W. O’Malley Jr, J. Cappello, H. Ghandehari. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. J. Control. Release 94:433–445 (2004).
K. Itaka, K. Yamauchi, A. Harada, K. Nakamura, H. Kawaguchi, and K. Kataoka. Polyion complex micelles from plasmid DNA and poly(ethylene glycol)-poly(l-lysine) block copolymer as serum-tolerable polyplex system: physicochemical properties of micelles relevant to gene transfection efficiency. Biomaterials 24:4495–4506 (2003).
D. Fischer, T. Bieber, Y. Li, H. P. Elsasser and T. Kissel. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 16:1273–1279 (1999).
P. van de Wetering, E. E. Moret, N. M. Schuurmans-Nieuwenbroek, M. J. van Steenbergen, and W. E. Hennink. Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjug. Chem. 10:589–597 (1999).
D. E. Meyer, and A. Chilkoti. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules 3:357–367 (2002).
D. W. Lim, K. Trabbic-Carlson, J. A. Mackay, and A. Chilkoti. Improved non-chromatographic purification of a recombinant protein by cationic elastin-like polypeptides. Biomacromolecules 8(5):1417–1424 (2007).
C. Plank, M. X. Tang, A. R. Wolfe, and F. C. Szoka Jr. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 10:319–332 (1999).
D. Fischer, H. Dautzenberg, K. Kunath, and T. Kissel. Poly(diallyldimethylammonium chlorides) and their N-methyl-N-vinylacetamide copolymer-based DNA-polyplexes: role of molecular weight and charge density in complex formation, stability, and in vitro activity. Int. J. Pharm. 280:253–269 (2004).
D. T. McPherson, J. Xu, and D. W. Urry. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr. Purif. 7:51–57 (1996).
D. E. Meyer, and A. Chilkoti. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 17:1112–1115 (1999).
J. Cappello, J. Crissman, M. Dorman, M. Mikolajczak, G. Textor, M. Marquet, and F. Ferrari. Genetic engineering of structural protein polymers. Biotechnol. Prog. 6:198–202 (1990).
D. W. Urry, T. M. Parker, M. C. Reid, and D. C. Gowda. Biocompatibility of the bioelastic materials, poly(GVGVP) and its γ-irradiation cross-linked matrix: summary of generic biological test results. J. Bioact. Compat. Poly. 6:263–282 (1991).
A. C. Rincon, I. T. Molina-Martinez, B. de Las Heras, M. Alonso, C. Bailez, J. C. Rodriguez-Cabello, and R. Herrero-Vanrell. Biocompatibility of elastin-like polymer poly(VPAVG) microparticles: in vitro and in vivo studies. J. Biomed. Mater. Res. A 78:343–351 (2006).
D. W. Urry. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J. Phys. Chem. B 101:11007–11028 (1997).
A. Girotti, J. Reguera, F. J. Arias, M. Alonso, A. M. Testera, and J. C. Rodríguez-Cabello. Influence of the molecular weight on the inverse temperature transition of a model genetically engineered elastin-like pH-responsive polymer. Macromolecules 37:3396–3400 (2004).
M. R. Dreher, D. Raucher, N. Balu, O. Michael Colvin, S. M. Ludeman and A. Chilkoti. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J. Control. Release 91:31–43 (2003).
D.Y. Furgeson, M. R. Dreher, and A. Chilkoti. Structural optimization of a “smart” doxorubicin–polypeptide conjugate for thermally targeted delivery to solid tumors. J. Control. Release 110:362–369 (2006).
W. Liu, M. R. Dreher, D. Y. Furgeson, K. V. Peixoto, H. Yuan, M. R. Zalutsky, and A. Chilkoti. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J. Control. Release 116:170–178 (2006).
D. Raucher, and A. Chilkoti. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 61:7163–7170 (2001).
G. L. Bidwell 3rd, and D. Raucher. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol. Cancer. Ther. 4:1076–1085 (2005).
I. Massodi, G. L. Bidwell 3rd, and D. Raucher. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J. Control. Release 108:396–408 (2005).
R. Langer, and D. A. Tirrell. Designing materials for biology and medicine. Nature 428:487–492 (2004).
A. Aris, J. X. Feliu, A. Knight, C. Coutelle, and A. Villaverde. Exploiting viral cell-targeting abilities in a single polypeptide, non-infectious, recombinant vehicle for integrin-mediated DNA delivery and gene expression. Biotechnol. Bioeng. 68:689–696 (2000).
L.K. Medina-Kauwe, M. Maguire, N. Kasahara, and L. Kedes. Nonviral gene delivery to human breast cancer cells by targeted Ad5 penton proteins. Gene Ther. 8:1753–1761 (2001).
A. Hatefi, Z. Megeed, and H. Ghandehari. Recombinant polymer–protein fusion: a promising approach towards efficient and targeted gene delivery. J. Gene Med. 8:468–476 (2006).
M. Haider, V. Leung, F. Ferrari, J. Crissman, J. Powell, J. Cappello, and H. Ghandehari. Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization. Mol. Pharm. 2:139–150 (2005).
A. Hatefi, J. Cappello, and H. Ghandehari. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm. Res. 24:773–779 (2007).
A. Zintchenko, M. Ogris, and E. Wagner. Temperature dependent gene expression induced by PNIPAM_based copolymers: potential of hyperthermia in gene transfer. Bioconjug. Chem. 17:766–772 (2006).
H. S. Bisht, D. S. Manickam, Y. You, and D. Oupicky. Temperature-controlled properties of DNA complexes with poly(ethylenimine)-graft-poly(N-isopropylacrylamide). Biomacromolecules 7:1169–1178 (2006).
P.H. Hirel, M. J. Schmitter, P. Dessen, G. Fayat, and S. Blanquet. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc. Natl. Acad. Sci. U. S. A. 86:8247–8251 (1989).
D. W. Urry. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 57:23–57 (1992).
A.M. Ponce, Z. Vujaskovic, F. Yuan, D. Needham and M. W. Dewhirst. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperthermia 22:205–213 (2006).
M. Neu, J. Sitterberg, U. Bakowsky, and T. Kissel. Stabilized nanocarriers for plasmids based upon cross-linked poly(ethylene imine). Biomacromolecules 7:3428–3438 (2006).
D. Putnam, C. A. Gentry, D. W. Pack, and R. Langer. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. U. S. A. 98:1200–1205 (2001).
K. L. Kiick, E. Saxon, D. A. Tirrell, and C. R. Bertozzi. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl. Acad. Sci. U.S.A. 99:19–24 (2002).
ACKNOWLEDGEMENTS
This work was funded by University of Wisconsin-Madison start-up funds to DYF. The ELP(1–30) gene was donated by Prof. Ashutosh Chilkoti (Duke University). We thank Prof. Glen Kwon (University of Wisconsin—Madison) for use of the NICOMP DLS; Prof. Maureen Barr (University of Wisconsin—Madison) for microscope access; and the UW Biotechnology Center for performing MALDI-TOF mass spectrum analysis. We express our thanks to Ms. Tracy P. Williamson for her critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, TH.H., Bae, Y. & Furgeson, D.Y. Intelligent Biosynthetic Nanobiomaterials (IBNs) for Hyperthermic Gene Delivery. Pharm Res 25, 683–691 (2008). https://doi.org/10.1007/s11095-007-9382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9382-5