Abstract
Purpose
This study evaluated the in vivo performance of a liposome formulation that co-encapsulates iohexol and gadoteridol as a multimodal contrast agent for computed tomography (CT) and magnetic resonance (MR)-based image guidance applications.
Materials and Methods
The pharmacokinetics and biodistribution studies were conducted in Balb-C mice using high performance liquid chromatography (HPLC) and inductively coupled plasma atomic emission spectrometry (ICP-AES) to detect iohexol and gadoteridol concentrations. The imaging efficacy of this liposome system was assessed in New Zealand White rabbits using a clinical CT and a clinical 1.5 Tesla MR scanner.
Results
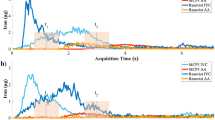
The vascular half-lives of the liposome encapsulated iohexol and gadoteridol in mice were found to be 18.4 ± 2.4 and 18.1 ± 5.1 h. When administered at the same dose the distribution (α phase) half-lives for the free contrast agents were 12.3 ± 0.5 min (iohexol) and 7.6 ± 0.9 min (gadoteridol); while, the elimination (β phase) half-lives were 3.0 ± 0.9 h for free iohexol and 3.0 ± 1.3 h for free gadoteridol. The CT and MR signal increases were measured and correlated with the concentrations of iohexol and gadoteridol detected in plasma samples.
Conclusion
The long in vivo circulation lifetime and simultaneous CT and MR signal enhancement provided by this liposome system make it a promising agent for image guidance applications.





Similar content being viewed by others
References
W.A. Kalender. X-ray based imaging for computer-assisted surgery. Minim. Invasive Ther. Allied Technol. 12: 52–58 (2003).
R. B. Sequeiros, R. Ojala, J. Kariniemi, J. Perala, J. Niinimaki, H. Reinikainen, and O. Tervonen. MR-guided interventional procedures: a review. Acta Radiol. 46: 576–586 (2005).
T. M. Peters. Image-guidance for surgical procedures. Phys. Med. Biol. 51: R505–540 (2006).
M. A. Rafferty, J. H. Siewerdsen, Y. Chan, M. J. Daly, D. J. Moseley, D. A. Jaffray, and J. C. Irish. Intraoperative cone-beam CT for guidance of temporal bone surgery. Otolaryngol. Head Neck Surg. 134: 801–808 (2006).
M. Uematsu, M. Sonderegger, A. Shioda, K. Tahara, T. Fukui, Y. Hama, T. Kojima, J. R. Wong, and S. Kusano. Daily positioning accuracy of frameless stereotactic radiation therapy with a fusion of computed tomography and linear accelerator (focal) unit: evaluation of z-axis with a z-marker. Radiother. Oncol. 50: 337–339 (1999).
D. A. Jaffray, J. H. Siewerdsen, J. W. Wong, and A. A. Martinez. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 53: 1337–1349 (2002).
A. K. Exadaktylos, J. Duwe, F. Eckstein, C. Stoupis, H. Schoenfeld, H. Zimmermann, and T. P. Carrel. The role of contrast-enhanced spiral CT imaging versus chest X-rays in surgical therapeutic concepts and thoracic aortic injury: a 29-year Swiss retrospective analysis of aortic surgery. Cardiovasc. J. S. Afr. 16: 162–165 (2005).
M. Saeed, D. Saloner, O. Weber, A. Martin, C. Henk, and C. Higgins. MRI in guiding and assessing intramyocardial therapy. Eur. Radiol. 15: 851–863 (2005).
J. G. Rosenman, E. P. Miller, G. Tracton, and T. J. Cullip. Image registration: an essential part of radiation therapy treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 40: 197–205 (1998).
P. Leander, P. Hoglund, A. Borseth, Y. Kloster, and A. Berg. A new liposomal liver-specific contrast agent for CT: first human phase-I clinical trial assessing efficacy and safety. Eur. Radiol. 11: 698–704 (2001).
C. Y. Kao, E. A. Hoffman, K. C. Beck, R. V. Bellamkonda, and A. V. Annapragada. Long-residence-time nano-scale liposomal iohexol for X-ray-based blood pool imaging. Acad. Radiol. 10: 475–483 (2003).
S. Erdogan, A. Roby, R. Sawant, J. Hurley, and V. P. Torchilin. Gadolinium-loaded polychelating polymer-containing cancer cell-specific immunoliposomes. J. Liposome Res. 16: 45–55 (2006).
K. F. Pirollo, J. Dagata, P. Wang, M. Freedman, A. Vladar, S. Fricke, L. Ileva, Q. Zhou, and E. H. Chang. A tumor-targeted nanodelivery system to improve early MRI detection of cancer. Mol. Imaging 5: 41–52 (2006).
W. J. Mulder, G. J. Strijkers, G. A. van Tilborg, A. W. Griffioen, and K. Nicolay. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 19: 142–164 (2006).
S. Mukundan, Jr., K. B. Ghaghada, C. T. Badea, C. Y. Kao, L. W. Hedlund, J. M. Provenzale, G. A. Johnson, E. Chen, R. V. Bellamkonda, and A. Annapragada. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am. J. Roentgenol. 186: 300–307 (2006).
M. S. Martina, J. P. Fortin, C. Menager, O. Clement, G. Barratt, C. Grabielle-Madelmont, F. Gazeau, V. Cabuil, and S. Lesieur. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 127: 10676–10685 (2005).
C. M. Moran, J. A. Ross, C. Cunningham, M. Butler, T. Anderson, D. Newby, K. A. Fox, and W. N. McDicken. Manufacture and acoustical characterisation of a high-frequency contrast agent for targeting applications. Ultrasound Med. Biol. 32: 421–428 (2006).
J. Zheng, G. Perkins, A. Kirilova, C. Allen, and D. A. Jaffray. Multimodal contrast agent for combined computed tomography and magnetic resonance imaging applications. Invest. Radiol. 41: 339–348 (2006).
M. T. Krauze, J. Forsayeth, J. W. Park, and K. S. Bankiewicz. Real-time imaging and quantification of brain delivery of liposomes. Pharm. Res. (2006).
M. Vaccaro, A. Accardo, D. Tesauro, G. Mangiapia, D. Lof, K. Schillen, O. Soderman, G. Morelli, and L. Paduano. Supramolecular aggregates of amphiphilic gadolinium complexes as blood pool MRI/MRA contrast agents: physicochemical characterization. Langmuir 22: 6635–6643 (2006).
H. Y. Lee, H. W. Jee, S. M. Seo, B. K. Kwak, G. Khang, and S. H. Cho. Diethylenetriaminepentaacetic acid-gadolinium (DTPA-Gd)-conjugated polysuccinimide derivatives as magnetic resonance imaging contrast agents. Bioconjug. Chem. 17: 700–706 (2006).
A. Accardo, D. Tesauro, P. Roscigno, E. Gianolio, L. Paduano, G. D’Errico, C. Pedone, and G. Morelli. Physicochemical properties of mixed micellar aggregates containing CCK peptides and Gd complexes designed as tumor specific contrast agents in MRI. J. Am. Chem. Soc. 126: 3097–3107 (2004).
V. P. Torchilin. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv. Drug Deliv. Rev. 54: 235–252 (2002).
V. P. Torchilin, M. D. Frank-Kamenetsky, and G. L. Wolf. CT visualization of blood pool in rats by using long-circulating, iodine-containing micelles. Acad. Radiol. 6: 61–65 (1999).
D. Zhu, R. D. White, P. A. Hardy, N. Weerapreeyakul, K. Sutthanut, and M. Jay. Biocompatible nanotemplate-engineered nanoparticles containing gadolinium: stability and relaxivity of a potential MRI contrast agent. J. Nanosci. Nanotechnol. 6: 996–1003 (2006).
I. R. Corbin, H. Li, J. Chen, S. Lund-Katz, R. Zhou, J. D. Glickson, and G. Zheng. Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents. Neoplasia 8: 488–498 (2006).
M. A. McDonald and K. L. Watkin. Investigations into the physicochemical properties of dextran small particulate gadolinium oxide nanoparticles. Acad. Radiol. 13: 421–427 (2006).
H. Lee, E. Lee, K. Kim do, N. K. Jang, Y. Y. Jeong, and S. Jon. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J. Am. Chem. Soc. 128: 7383–7389 (2006).
J. F. Hainfeld, D. N. Slatkin, T. M. Focella, and H. M. Smilowitz. Gold nanoparticles: a new X-ray contrast agent. Br. J. Radiol. 79: 248–253 (2006).
O. Rabin, J. Manuel Perez, J. Grimm, G. Wojtkiewicz, and R. Weissleder. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat. Mater. 5: 118–122 (2006).
V. S. Talanov, C. A. Regino, H. Kobayashi, M. Bernardo, P. L. Choyke, and M. W. Brechbiel. Dendrimer-based nanoprobe for dual modality magnetic resonance and fluorescence imaging. Nano Lett. 6: 1459–1463 (2006).
H. Kobayashi and M. W. Brechbiel. Nano-sized MRI contrast agents with dendrimer cores. Adv. Drug Deliv. Rev. 57: 2271–2286 (2005).
S. Langereis, Q. G. de Lussanet, M. H. van Genderen, E. W. Meijer, R. G. Beets-Tan, A. W. Griffioen, J. M. van Engelshoven, and W. H. Backes. Evaluation of Gd(III)DTPA-terminated poly(propylene imine) dendrimers as contrast agents for MR imaging. NMR Biomed. 19: 133–141 (2006).
Y. Fu, D. E. Nitecki, D. Maltby, G. H. Simon, K. Berejnoi, H. J. Raatschen, B. M. Yeh, D. M. Shames, and R. C. Brasch. Dendritic iodinated contrast agents with PEG-cores for CT imaging: synthesis and preliminary characterization. Bioconjug. Chem. 17: 1043–1056 (2006).
M. Port, C. Corot, X. Violas, P. Robert, I. Raynal, and G. Gagneur. How to compare the efficiency of albumin-bound and nonalbumin-bound contrast agents in vivo: the concept of dynamic relaxivity. Invest. Radiol. 40: 565–573 (2005).
Y. Zhang, P. L. Choyke, H. Lu, H. Takahashi, R. B. Mannon, X. Zhang, H. Marcos, K. C. Li, and J. B. Kopp. Detection and localization of proteinuria by dynamic contrast-enhanced magnetic resonance imaging using MS325. J. Am. Soc. Nephrol. 16: 1752–1757 (2005).
A. M. Morawski, G. A. Lanza, and S. A. Wickline. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr. Opin. Biotechnol. 16: 89–92 (2005).
M. A. McDonald and K. L. Watkin. Small particulate gadolinium oxide and gadolinium oxide albumin microspheres as multimodal contrast and therapeutic agents. Invest. Radiol. 38: 305–310 (2003).
M. F. Kircher, U. Mahmood, R. S. King, R. Weissleder, and L. Josephson. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 63: 8122–8125 (2003).
G. R. Norman and D. L. Streiner. Biostatistics: The Bare Essentials. B. C. Decker, Hamilton, Canada, 2000.
W. Mutzel and U. Speck. Pharmacokinetics and biotransformation of iohexol in the rat and the dog. Acta Radiol. Suppl. 362: 87–92 (1980).
M. F. Tweedle, X. Zhang, M. Fernandez, P. Wedeking, A. D. Nunn, and H. W. Strauss. A noninvasive method for monitoring renal status at bedside. Invest. Radiol. 32: 802–805 (1997).
M. Heilmann, F. Kiessling, M. Enderlin, and L. R. Schad. Determination of pharmacokinetic parameters in DCE MRI: consequence of nonlinearity between contrast agent concentration and signal intensity. Invest. Radiol. 41: 536–543 (2006).
S. J. McLachlan, S. Eaton, and D. N. De Simone. Pharmacokinetic behavior of gadoteridol injection. Invest. Radiol. 27(Suppl 1): S12–S15 (1992).
A. Arvidsson and A. Hedman. Plasma and renal clearance of iohexol—a study on the reproducibility of a method for the glomerular filtration rate. Scand. J. Clin. Lab. Invest. 50: 757–761 (1990).
M. F. Tweedle. The ProHance story: the making of a novel MRI contrast agent. Eur. Radiol. 7(Suppl 5): 225–230 (1997).
C. Rasch, I. Barillot, P. Remeijer, A. Touw, M. van Herk, and J. V. Lebesque. Definition of the prostate in CT and MRI: a multi-observer study. Int. J. Radiat. Oncol. Biol. Phys. 43: 57–66 (1999).
H. U. Kauczor. Multimodal imaging and computer assisted diagnosis for functional tumour characterisation. Cancer Imaging 5: 46–50 (2005).
P. L. Choyke. Contrast agents for imaging tumor angiogenesis: is bigger better? Radiology 235: 1–2 (2005).
M. R. Dreher, W. Liu, C. R. Michelich, M. W. Dewhirst, F. Yuan, and A. Chilkoti. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 98: 335–344 (2006).
K. A. Miles. Functional computed tomography in oncology. Eur. J. Cancer 38: 2079–2084 (2002).
H. E. Daldrup-Link, G. H. Simon, and R. C. Brasch. Imaging of tumor angiogenesis: current approaches and future prospects. Curr. Pharm. Des. 12: 2661–2672 (2006).
Acknowledgements
This work is funded in-part by a CIHR Operating Grant and a CIHR Proof of Principle Grant to D.A. Jaffray and C. Allen, the Premier’s Research Excellence Award, the Fidani Chair in Radiation Physics and the Grange Advanced Simulation Initiative. J. Zheng is grateful for the Excellence in Radiation Research for the 21st Century Training Fellowship and the Mitchell Scholarship. The authors would like to thank the UHN animal care staff for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, J., Liu, J., Dunne, M. et al. In Vivo Performance of a Liposomal Vascular Contrast Agent for CT and MR-Based Image Guidance Applications. Pharm Res 24, 1193–1201 (2007). https://doi.org/10.1007/s11095-006-9220-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9220-1