Purpose
Spray-drying process was used for the development of dried polymeric nanocapsules. The purpose of this research was to investigate the effects of formulation and process variables on the resulting powder characteristics in order to optimize them.
Materials and Methods
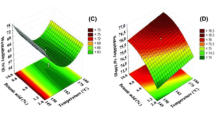
Experimental designs were used in order to estimate the influence of formulation parameters (nanocapsules and silica concentrations) and process variables (inlet temperature, spray-flow air, feed flow rate and drying air flow rate) on spray-dried nanocapsules when using silica as drying auxiliary agent. The interactions among the formulation parameters and process variables were also studied. Responses analyzed for computing these effects and interactions were outlet temperature, moisture content, operation yield, particles size, and particulate density. Additional qualitative responses (particles morphology, powder behavior) were also considered.
Results
Nanocapsules and silica concentrations were the main factors influencing the yield, particulate density and particle size. In addition, they were concerned for the only significant interactions occurring among two different variables. None of the studied variables had major effect on the moisture content while the interaction between nanocapsules and silica in the feed was of first interest and determinant for both the qualitative and quantitative responses. The particles morphology depended on the feed formulation but was unaffected by the process conditions.
Conclusion
This study demonstrated that drying nanocapsules using silica as auxiliary agent by spray drying process enables the obtaining of dried micronic particle size. The optimization of the process and the formulation variables resulted in a considerable improvement of product yield while minimizing the moisture content.



Similar content being viewed by others
References
P. Couvreur, C. Dubernet, and F. Puisieux. Controlled drug delivery with nanoparticles: current possibilities and future trends. Eur. J. Pharm. Biopharm. 41:2–13 (1995).
M. D. Coffin and J. W. McGinity. Biodegradable pseudolatexes: the chemical stability of poly (D, L-Lactide) and poly(ɛ-caprolactone) nanoparticles in aqueous media. Pharm. Res. 9:200–205 (1992).
B. Magenheim and S. Benita. Nanoparticle characterization: a comprehensive physicochemical approach. S.T.P. Pharm. Sci. 1:221–241 (1991).
E. Allémann, R. Gurny, and E. Doelker. Drug-loaded nanoparticles—preparation methods and drug targeting issues. Eur. J. Pharm. Biopharm. 39:173–191 (1993).
W. Abdelwahed, G. Degobert, and H. Fessi. Freeze-drying of nanocapsules: impact of annealing on the drying process. Int. J. Pharm. 324:74–82 (2006).
W. Abdelwahed, G. Degobert, and H. Fessi. Investigation of nanocapsules stabilization by amorphous excipients during freeze drying and storage. Eur. J. Pharm. Biopharm. 63:87–94 (2006).
W. Abdelwahed, G. Degobert, and H. Fessi. A pilot study of freeze drying of poly (epsilon-caprolactone) nanocapsules stabilized by poly (vinyl alcohol): formulation and process optimization. Int. J. Pharm. 309:178–188 (2006).
C. R. Müller, V. L. Bassani, A. R. Pohlmann, C. B. Michalowski, P. R. Petrovick, and S. S. Guterres. Preparation and characterization of spray-dried nanocapsules. Drug Dev. Ind. Pharm. 26:343–347 (2000).
K. Master. Spray Drying Handbook. Longman Scientific and Technical, New York, 1991.
J. Broadhead, S. K. Edmond Rouan, and C. T. Rhodes. The spray drying of pharmaceuticals. Drug. Dev. Ind. Pharm. 18:1169–1206 (1992).
K. G. H. Desai and H. J. Park. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J. Microencap. 22:179–192 (2005).
V. R. Sinha, R. Anitha, S. Ghosh, A. Nanda, and R. Kumria. Complexation of celecoxib with beta-cyclodextrin: characterization of the interaction in solution and in solid state. J. Pharm. Sci. 94:676–687 (2005).
B. Boh, E. Knez, and M. Staresinic. Microencapsulation of higher hydrocarbon phase change materials by in situ polymerization. J. Microencap. 22:715–735 (2005).
D. E. Oakley. Produce uniform particles by spray drying. Chem. Eng. Prog. 93:48–54 (1997).
U. Conte, B. Conti, P. Giunchedi, and L. Maggi. Spray dried polylactide microsphere preparation: influence of the technological parameters. Drug Dev. Ind. Pharm. 20:253–258 (1994).
S. Wendel and M. Celik. An overview of spray-drying applications. Pharm. Technol. 10:124–156 (1997).
P. Tewa-Tagne, S. Briançon, and H. Fessi. Spray-dried microparticles containing polymeric nanocapsules: formulation aspects, liquid phase interactions and particle characteristics. Int. J. Pharm. 325:63–74 (2006).
H. Fessi, F. Puisieux, and J. P. Devissaguet. Procédé de préparation de systèmes colloïdaux dispersibles d’une substance sous forme de nanocapsules. Eur. Pat. 0274961 B1 (1992).
G. E. P. Box, W. G. Hunter, J. S. Hunter, and W. G. Hunter. Statistics for Experimenters. Wiley, New York, 1978.
I. Montasser, S. Briançon, J. Lieto, and H. Fessi. Méthodes d’obtention et mécanismes de formation de nanoparticules polymériques. J. Pharm. Belg. 55:155–167 (2000).
R. C. Rowe, P. J. Sheskey, and P. J. Weller. Handbook of Pharmaceutical Excipients, 4th ed. Pharmaceutical, London, 2003.
A. R. Pohlmann, V. Weiss, O. Mertins, N. Pesce da Silveira, and S. S. Guterres. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: development, stability evaluation and nanostructure models. Eur. J. Pharm. Sci. 16:305–312 (2002).
S. S. Guterres, C. R. Muller, C. B. Michalowski, A. R. Pohlmann, and T. Dalla Costa. Gastro-intestinal tolerance following oral administration of spray-dried diclofenac-loaded nanocapsules and nanospheres. S.T.P. Pharma. Sci. 11:229–233 (2001).
S. R. Raghavan and S. A. Khan. Shear-thickening response of fumed silica suspensions under steady and oscillatory shear. J. Coll. Int. Sci. 185:57–67 (1997).
F. Yziquel, P. J. Carreau, and P. A. Tanguy. Non linear viscoelastic behavior of fumed silica suspension. Rheol. Acta 38:14–25 (1999).
J. Forsman, J. P. Harrison and A. Rutenberg. Elasticity of a percolation system: silica smoke. Can. J. Phys. 65:767–771 (1987).
M. A. Goula and K. G. Adamopoulos. Spray-drying of tomato pulp in dehumidified air. I. The effect on product recovery. J. Food Eng. 66:25–34 (2005).
F Pavanetto, I. Genta, P. Giunchedi, B. Conti, and U. Conte. Spray dried albumin microspheres for the intra-articular delivery of dexamethazone. J. Microencap 11:445–454 (1994).
P. Giunchedi, C. Juliano, E. Gavini, M. Cossu, and M. Sorrenti. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur. J. Pharm. Biopharm. 53:233–239 (2002).
A. Martinac, J. Filipovic -Grcic´, B. Perissutti, D. Voinovich, and Z. Pavelic. Spray-dried chitosan/ethylcellulose microspheres for nasal drug delivery: swelling study and evaluation of in vitro drug release properties. J. Microencap 22:549–561 (2005).
K. B. Prinn, R. H. Costantino, and M. Tracy. Statistical modeling of protein spray drying at the lab scale. AAPS Pharm. Sci. Tech. 3:1–8 (2002).
A. Billon, B. Bataille, G. Cassanas, and M. Jacob. Development of spray-dried acetaminophen microparticles using experimental designs. Int. J. Pharm. 203:159–168 (2000).
G. F. Palmieri, P. Wehrlé, and A. Stamm. Evaluation of spray-drying as a method to prepare microparticles for controlled drug release. Drug Dev. Ind. Pharm. 20:2859–2879 (1994).
K. Mosen, K. Backstrom, K. Thalberg, T. Schaefer, H. G. Kristensen, and A. Axelsoon. Particle formation and capture during spray drying of inhalable particles. Pharm. Dev. Tech. 9:409–417 (2004).
S. Nath and G. R. Satpathy. A systematic approach for investigation of spray drying processes. Drying Tech. 16:1173–1193 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tewa-Tagne, P., Degobert, G., Briançon, S. et al. Spray-drying Nanocapsules in Presence of Colloidal Silica as Drying Auxiliary Agent: Formulation and Process Variables Optimization Using Experimental Designs. Pharm Res 24, 650–661 (2007). https://doi.org/10.1007/s11095-006-9182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9182-3