Abstract
Purpose
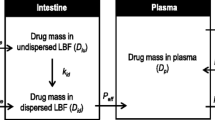
To examine the correlation between the in vitro solubilization process of lipophilic compounds from different lipid solutions and the corresponding in vivo oral bioavailability data. In particular, to assess the influence of intra-enterocyte processes (metabolism and lymphatic absorption) on this correlation.
Materials and Methods
The dissolution of progesterone and vitamin D3 in long (LCT), medium (MCT) and short (SCT) chain triglyceride solutions were tested in a dynamic in vitro lipolysis model. The absolute oral bioavailability of the drugs from the tested formulations was investigated in rats. Vitamin D3 bioavailability was also examined following lymphatic transport blockage induced by cycloheximide (3 mg/kg).
Results
The dynamic in vitro lipolysis experiments indicated a rank order of MCT > LCT > SCT for both progesterone and vitamin D3. The bioavailability of progesterone correlated with the in vitro data, despite its significant pre-systemic metabolism. For vitamin D3, an in vivo performance rank order of LCT > MCT > SCT was obtained. However, when the lymphatic transport was blocked the bioavailability of vitamin D3 correlated with in vitro data.
Conclusions
The in vitro lipolysis model is useful for optimization of oral lipid formulations even in the case of pre-systemic metabolism in the gut. However, when lymphatic transport is a significant route of absorption, the in vitro lipolysis data may not be predictive for actual in vivo absorption.







Similar content being viewed by others
References
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3–26 (2001).
C. W. Pouton. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and self-microemulsifying drug delivery systems. Eur. J. Pharm Sci. 11:S93–S98 (2000).
A. J. Humberstone, and W. N. Charman. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 25:103–128 (1997).
J. M. Dintaman, and J. A. Silverman. Inhibition of P-glycoprotein by d-á-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm Res. 16:1550–1556 (1999).
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
C. J. H. Porter, and W. N. Charman. In vitro assessment of oral lipid based formulations. Adv. Drug Deliv. Rev. 50:S127–S147 (2001).
J. O. Christensen, K. Schultz, B. Mollgaard, H. G. Kristensen, and A. Mullertz. Solubilisation of poorly water-soluble drugs during in vitro lipolysis of medium- and long-chain triacylglycerols. Eur. J. Pharm Sci. 23:287–296 (2004).
G. A. Kossena, B. J. Boyd, Christopher J. H. Porter, and W. N. Charman. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J. Pharm Sci. 92:634–648 (2003).
H. Ljusberg-Wahren, F. Seier Nielsen, M. Brogard, E. Troedsson, and A. Mullertz. Enzymatic characterization of lipid-based drug delivery systems. Int. J. Pharm 298:328–332 (2005).
W. Nienstedt, A. Ojanotko, and H. Toivonen. Metabolism of progesterone, 17-hydroxyprogesterone and deoxycorticosterone by human small intestine in vitro. J. Steroid Biochem. 13:1417–1420 (1980).
M. P. Harri, W. Nienstedt, and K. Hartiala. Steroid metabolism by the canine intestine. II. The metabolism of progesterone by the jejunal mucosa in vitro. Scand J. Gastroenterol 5:415–419 (1970).
A. Dahan, and A. Hoffman. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur. J. Pharm Sci. 24:381–388 (2005).
L. Sek, C. J. Porter, A. M. Kaukonen, and W. N. Charman. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J. Pharm Pharmacol 54:29–41 (2002).
L. Sek, C. J. H. Porter, and W. N. Charman. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situ densitometric analysis. J. Pharml. Biomed. Anal. 25:651–661 (2001).
A. Hoffman, and G. Levy. Kinetics of drug action in disease states. XXIX. Effect of experimental nephrotic syndrome on the pharmacodynamics of heptabarbital: implications of severe hypoalbuminemia. J. Pharmacol Exp. Ther. 249:117–122 (1989).
D. B. Gomis, V. J. Escotet Arias, L. E. Fidalgo Alvarez, and M. D. Gutierrez Alvarez. Simultaneous determination of vitamins D3, E and K1 and retinyl palmitate in cattle plasma by liquid chromatography with a narrow-bore column. J. Chromatogr. B Biomed. Appl. 660:49–55 (1994).
C. J. H. Porter, A. M. Kakunen, A. Taillardat-Bertschinger, B. J. Boyd, J. M. O'Connor, G. A. Edwards, and W. N. Charman. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J. Pharm Sci. 93:1110–1121 (2004).
A. Bernard, and H. Carlier. Intestinal lymphatic absorption of labelled oleic acid in the normal rat and rat treated with actidione-cycloheximide or acetoxycycloheximide. C R Seances Acad Sci. III 292:97–100 (1981).
S. M. Sabesin, and K. J. Isselbacher. Protein synthesis inhibition: mechanism for the production of impaired fat absorption. Science 147:1149–51 (1965).
T. Gershanik, and S. Benita. Positively charged self-emulsifying oil formulation for improving oral bioavailability of progesterone. Pharm Dev. Technol. 1:147–57 (1996).
K. J. MacGregor, J. K. Embleton, J. E. Lacy, E. A. Perry, L. J. Solomon, H. Seager, and C. W. Pouton. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv. Drug Deliv. Rev. 25:33–46 (1997).
I. Holmberg, L. Aksnes, T. Berlin, B. Lindback, J. Zemgals, and B. Lindeke. Absorption of a pharmacological dose of vitamin D3 from two different lipid vehicles in man: comparison of peanut oil and a medium chain triglyceride. Biopharm Drug Dispos 11:807–815 (1990).
A. Kuksis. Absorption of fat soluble vitamins. In A. Kuksis (ed.), Fat absorption, Vol. 2, CRC, Boca Raton, 1987, pp. 65–86.
S. M. Khoo, G. A. Edwards, C. J. H. Porter, and W. N. Charman. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J. Pharm Sci. 90:1599–1607 (2001).
K. J. Palin, and C. G. Wilson. The effect of different oils on the absorption of probucol in the rat. J. Pharm Pharmacol 36:641–3 (1984).
D. J. Hauss, S. E. Fogal, J. V. Ficorilli, C. A. Price, T. Roy, A. A. Jayaraj, and J. J. Keirns. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J. Pharm Sci. 87:164–169 (1998).
S. M. Caliph, W. N. Charman, and C. J. Porter. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J. Pharm Sci. 89:1073–84 (2000).
P. Gershkovich, and A. Hoffman. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur. J. Pharm Sci. 26:394–404 (2005).
Acknowledgments
This work is a part of Arik Dahan Ph.D dissertation. This study was supported by the Israeli Consortium of Pharmalogica. The authors thank Dr. Bashir Qadri and Inbal Yifrach for technical support, and Dr. Josh Backon and Pavel Gershkovich for constructive comments. A. Hoffman is affiliated with David R. Bloom Center of Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahan, A., Hoffman, A. Use of a Dynamic in Vitro Lipolysis Model to Rationalize Oral Formulation Development for Poor Water Soluble Drugs: Correlation with in Vivo Data and the Relationship to Intra-Enterocyte Processes in Rats. Pharm Res 23, 2165–2174 (2006). https://doi.org/10.1007/s11095-006-9054-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9054-x