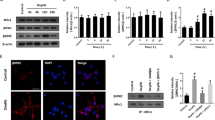
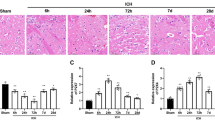
Abstract
Brain inflammation induced by ischemic stroke is an important cause of secondary brain injury. The nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and NLRP3 inflammasome signaling are believed to drive the progression of brain inflammation. Spermatogenesis-associated protein2 (SPATA2) functions as a partner protein that recruits CYLD, a negative regulator of NF-κB signaling, to signaling complexes. However, the role of SPATA2 in the central nervous system remains unclear and whether it is involved in regulating inflammatory responses remains controversial. Rats were subjected to transient middle cerebral artery occlusion followed by reperfusion (tMCAO/R) surgery. The expression and localization of SPATA2 in the brain were investigated. The lentivirus-mediated shRNA was employed to inhibit SPATA2 expression. The inflammatory responses and outcomes of Spata2 knockdown were investigated. SPATA2 was co-localized with CYLD in neurons. SPATA2 expression was reduced in tMCAO/R rats. Spata2 knockdown resulted in increased microglia, increased expression of Tnfa, Il-1β, and Il-18, decreased Garcia score, and increased infarct volume. Spata2 knockdown resulted in the activation of P38MAPK and NLRP3 inflammasome and the increased activation of NF-κB signaling. These results suggest that SPATA2 plays a protective role against brain inflammation induced by ischemia/reperfusion injury. Therefore, SPATA2 could be a potential therapeutic target for treating ischemic stroke.






Similar content being viewed by others
References
Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, Abd-Allah F, Abdelalim A, Abraha HN, Abu-Rmeileh NME et al (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):439–458
Johnson W, Onuma O, Owolabi M, Sachdev S (2016) Stroke: A global response is needed. Bull World Health Organ 94(9):634-634A
Heiss WD, Zaro Weber O (2017) Validation of MRI determination of the penumbra by PET measurements in ischemic stroke. J Nucl Med 58(2):187–193
Astrup J, Siesjo BK, Symon L (1981) Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 12(6):723–725
Fu Y, Liu Q, Anrather J, Shi F-D (2015) Immune interventions in stroke. Nat Rev Neurol 11(9):524–535
Shi K, Tian D-C, Li Z-G, Ducruet AF, Lawton MT, Shi F-D (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066
Xiong XY, Liu L, Yang QW (2016) Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 142:23–44
Gülke E, Mathias G, Tim M (2018) Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord 11:1756286418774254
Zhang DD, Zou MJ, Zhang YT, Fu WL, Xu T, Wang JX, Xia WR, Huang ZG, Gan XD, Zhu XM, Xu DG (2017) A novel IL-1RA-PEP fusion protein with enhanced brain penetration ameliorates cerebral ischemia-reperfusion injury by inhibition of oxidative stress and neuroinflammation. Exp Neurology 1(297):1–3
Works MG, Koenig JB, Sapolsky RM (2013) Soluble TNF receptor 1-secreting ex vivo-derived dendritic cells reduce injury after stroke. J Cereb Blood Flow Metab 33(9):1376–1385
Hosomi N, Ban CR, Naya T, Takahashi T, Kohno M (2005) Tumor necrosis factor-alpha neutralization reduced cerebral edema through inhibition of matrix metalloproteinase production after transient focal cerebral ischemia. J Cereb Blood Flow Metab 25(8):959–967
Touzani O, Boutin H, Lefeuvre R, Parker L, Rothwell N (2002) Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 Type I receptor. J Neurosci 22(1):38–43
Eldahshan W, Fagan SC, Ergul A (2019) Inflammation within the neurovascular unit: Focus on microglia for stroke injury and recovery. Pharmacol Res 147:104349
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171
Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, Brown GC (2013) Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci USA 110(43):E4098–E4107
Harari OA, Liao JK (2010) NF-κB and innate immunity in ischemic stroke. Ann NY Acad Sci 1207(1):32–40
Ridder DA, Schwaninger M (2009) NF-kappaB signaling in cerebral ischemia. Neuroscience 158(3):995–1006
Sun J, Nan G (2016) The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. J Mol Neurosci 59(1):90–98
Arthur JSC, Ley SC (2013) Mitogen-activated protein kinases in Inflammasomenate immunity. Nat Rev Immunol 13(9):679–692
Alishahi M, Farzaneh M, Ghaedrahmati F, Nejabatdoust A, Sarkaki A, Khoshnam SE (2019) NLRP3 inflammasome in ischemic stroke: As possible therapeutic target. Int J Stroke 14(6):574–591
Fann DY, Lim YA, Cheng YL, Lok KZ, Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, Chen CL, Arumugam TV (2018) Evidence that NF-kappaB and Mapk signaling promotes NLRP activation in neurons following ischemic stroke. Mol Neurobiol 55(2):1082–1096
Elliott PR, Leske D, Hrdinka M, Bagola K, Fiil BK, McLaughlin SH, Wagstaff J, Volkmar N, Christianson JC, Kessler BM, Freund SM, Komander D, Gyrd-Hansen M (2016) SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling. Mol Cell 63(6):990–1005
Mathis BJ, Lai Y, Qu C, Janicki JS, Cui T (2015) CYLD-mediated signaling and diseases. Curr Drug Targets 16(4):284–294
Courtois G (2008) Tumor suppressor CYLD: negative regulation of NF-κB signaling and more. Cell Mol Life Sci 65(7–8):1123
Jiang J, Luo Y, Qin W, Ma H, Li Q, Zhan J, Zhang Y (2017) Electroacupuncture suppresses the NF-kappaB signaling pathway by upregulating cylindromatosis to alleviate inflammatory injury in cerebral ischemia/reperfusion rats. Front Mol Neurosci 10:363
Zhao J, Zhao J, Xu G, Wang Z, Gao J, Cui S, Liu J (2017) Deletion of Spata2 by CRISPR/Cas9n causes increased inhibin alpha expression and attenuated fertility in male mice. Biol Reprod 97(3):497–513
Claudio Maran ET, Masola V, Onisto M (2009) The story of SPATA2 (spermatogenesis-associated Protein 2): from sertoli cells to pancreatic beta-cells. Curr Genom 10(5):361–363
Onisto M, Slongo LM, Graziotto R, Zotti L, Negro A, Merico M, Moro E, Foresta C (2001) Evidence for FSH-dependent upregulation of SPATA2 (spermatogenesis-associated protein 2). Biochem Biophys Res Commun 283(1):86–92
Wagner SA, Satpathy S, Beli P, Choudhary C (2016) SPATA2 links CYLD to the TNF-alpha receptor signaling complex and modulates the receptor signaling outcomes. EMBO J 35(17):1868–1884
Schlicher L, Wissler M, Preiss F, Brauns-Schubert P, Jakob C, Dumit V, Borner C, Dengjel J, Maurer U (2016) SPATA2 promotes CYLD activity and regulates TNF-induced NF-kappaB signaling and cell death. EMBO Rep 17(10):1485–1497
Kupka S, De Miguel D, Draber P, Martino L, Surinova S, Rittinger K, Walczak H (2016) SPATA2-mediated binding of CYLD to HOIP enables CYLD recruitment to signaling complexes. Cell Rep 16(9):2271–2280
Yang XD, Li W, Zhang S, Wu D, Jiang X, Tan R, Niu X, Wang Q, Wu X, Liu Z, Chen LF, Qin J, Su B (2020) PLK4 deubiquitination by Spata2-CYLD suppresses NEK7-mediated NLRP3 inflammasome activation at the centrosome. EMBO J 39(2):e102201
Wei R, Xu LW, Liu J, Li Y, Zhang P, Shan B, Lu X, Qian L, Wu Z, Dong K, Zhu H, Pan L, Yuan J, Pan H (2017) SPATA2 regulates the activation of RIPK1 by modulating linear ubiquitination. Genes Dev 31(11):1162–1176
Qin WY, Luo Y, Chen L, Tao T, Li Y, Cai YL, Li YH (2013) Electroacupuncture could regulate the NF-κB signaling pathway to ameliorate the inflammatory injury in focal cerebral ischemia/reperfusion model rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2013/924541
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke 26(4):627–635
Zhou X, Lu W, Wang Y, Li J, Luo Y (2020) A20-binding inhibitor of NF-kappaB 1 ameliorates neuroinflammation and mediates antineuroinflammatory effect of electroacupuncture in cerebral ischemia/reperfusion rats. Evid Based Complement Alternat Med 2020:6980398
Onisto M, Graziotto R, Scannapieco P, Marin P, Merico M, Slongo ML, Foresta C (2000) A novel gene (PD1) with a potential role on rat spermatogenesis. J Endocrinol Invest 23(9):605–608
Moro E, Maran C, Slongo ML, Argenton F, Toppo S, Onisto M (2007) Zebrafish spata2 is expressed at early developmental stages. Int J Dev Biol 51(3):241–246
Graziotto R, Foresta C, Scannapieco P, Zeilante P, Russo A, Negro A, Salmaso R, Onisto M (1999) cDNA cloning and characterization of PD1a novel human testicular protein with different expressions in various testiculopathies. Exp Cell Res 248(2):620–626
Cantarella G (2003) The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424(6950):801–805
Brummelkamp TR, Nijman SM, Dirac AM, Bernards R (2003) Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424(6950):797–801
Schaeffer V, Akutsu M, Olma MH, Gomes LC, Kawasaki M, Dikic I (2014) Binding of OTULIN to the PUB domain of HOIP controls NF-kappaB signaling. Mol Cell 54(3):349–361
Enesa K, Zakkar M, Chaudhury H, le Luong A, Rawlinson L, Mason JC, Haskard DO, Dean JL, Evans PC (2008) NF-kappaB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J Biol Chem 283(11):7036–7045
Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430(7000):694–699
Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G (2003) CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424(6950):793–796
Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, Orsolits B, Molnár G, Heindl S, Schwarcz AD (2020) Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367:528–537
Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A (2016) Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 7:11499
Luo P, Chu SF, Zhang Z, Xia CY, Chen NH (2019) Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain Res Bull 146:12–21
Gronhoj MH, Clausen BH, Fenger CD, Lambertsen KL, Finsen B (2017) Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain Behav Immun 65:296–311
Rothaug M, Becker-Pauly C, Rose-John S (2016) The role of interleukin-6 signaling in nervous tissue. Biochem Biophys Acta 1863(6):1218–1227
Tarkowski E (1995) Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 26(8):1393–1398
Lambertsen KL, Finsen B, Clausen BH (2019) Post-stroke inflammation-target or tool for therapy? Acta Neuropathol 137(5):693–714
Lambertsen KL, Biber K, Finsen B (2012) Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32(9):1677–1698
Gulyás B, Tóth M, Schain M, Airaksinen A, Vas Á, Kostulas K, Lindström P, Hillert J, Halldin C (2012) Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: a PET study with the TSPO molecular imaging biomarker [11C] vinpocetine. J Neurol Sci 320(1–2):110–117
Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665
Boraschi D, Italiani P, Weil S, Martin MU (2018) The family of the interleukin-1 receptors. Immunol Rev 281(1):197–232
Fields JK, Sebastian G, Sundberg EJ (2019) Structural basis of IL-1 family cytokine signaling. Front Immunol 10:1412
Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A, Lok KZ, Foo SL, Wang YC, Li YI, Drummond GR, Basta M, Magnus T, Jo DG, Mattson MP, Sobey CG, Arumugam TV (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4:e790
Wheeler RD, Boutin H, Touzani O, Luheshi GN, Takeda K, Rothwell NJ (2003) No role for interleukin-18 in acute murine stroke-induced brain injury. J Cereb Blood Flow Metab 23(5):531–535
Hao Y, Ding J, Hong R, Bai S, Wang Z, Mo C, Hu Q, Li Z, Guan Y (2019) Increased interleukin-18 level contributes to the development and severity of ischemic stroke. Aging 11(18):7457–7472
Stott DJ, Paul W, Ann R, Michele R, Ian F, Naveed S, Westendorp RGJ, Wouter JJ, Cobbe SM, Lowe GDO (2009) Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol 1:253
Salani PBF, Cacciari C, Picchetto L, Cao M, Bizzoni F, Rasura M (2009) Disease outcome, alexithymia and depression are differently associated with serum IL-18 levels in acute stroke. Curr Neurovasc Res 6(3):163–170
Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA (2004) Differences in signaling pathways by IL-1β and IL-18. Proc Natl Acad Sci 101(23):8815–8820
Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, Rieser E, Martino L, Rittinger K, Walczak H (2015) LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep 13(10):2258–2272
Takiuchi T, Nakagawa T, Tamiya H, Fujita H, Sasaki Y, Saeki Y, Takeda H, Sawasaki T, Buchberger A, Kimura T, Iwai K (2014) Suppression of LUBAC-mediated linear ubiquitination by a specific interaction between LUBAC and the deubiquitinases CYLD and OTULIN. Genes Cells 19(3):254–272
Elliott PR, Nielsen SV, Marco-Casanova P, Fiil BK, Keusekotten K, Mailand N, Freund SM, Gyrd-Hansen M, Komander D (2014) Molecular basis and regulation of OTULIN-LUBAC interaction. Mol Cell 54(3):335–348
Lork M, Verhelst K, Beyaert R (2017) CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different. Cell Death Differ 24(7):1172–1183
Liao Z, Zhang X, Song C, Lin W, Cheng Y, Xie Z, Chen S, Nie Y, Li A, Zhang H, Li H, Li H, Xie Q (2020) ALV-J inhibits autophagy through the GADD45beta/MEKK4/P38MAPK signaling pathway and mediates apoptosis following autophagy. Cell Death Dis 11(8):684
Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-κB p65 subunit by mitogen-and stress-activated protein kinase-1 (MSK1). EMBO J 22(6):1313–1324
Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, Smaele ED, Tang W-J, D’Adamio L, Franzoso G (2004) Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol 6(2):146–153
Smaele ED, Zazzeroni F, Papa S, Nguyen DU, Franzoso G (2001) Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414(6861):308–313
Silvia A, Claudia M, Cristina B, Manuel SA, Giovanna R (2014) Interleukin 18 activates MAPKs and STAT3 but not NF-κB in hippocampal HT-22 cells. Brain Behav Immun 40:85–94
Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Silliman CC (2002) Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol 72(2):401–409
Kalina U, Kauschat D, Koyama N, Nuernberger H, Ballas K, Koschmieder S, Bug G, Hofmann WK, Hoelzer D, Ottmann OG (2000) IL-18 activates STAT3 in the natural killer cell line 92, augments cytotoxic activity, and mediates IFN-γ production by the stress kinase p38 and by the extracellular regulated kinases p44erk-1 and p42erk-21. J Immunol 165(3):1307–1313
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 30470606) and the Natural Science Foundation of Chongqing, Grand No. cstc2019jcyj-msxmX0630, to JJ.
Author information
Authors and Affiliations
Contributions
Conceptualization, YR and JJ; methodology, YR, JJ, and WJ; software, XZ and WL; validation, YR, JJ, and JW; investigation, YR, JJ, and XZ; data curation, YR, JJ, and WJ; writing—original draft preparation, YR; writing—review and editing, JJ; supervision, YL; project administration, YL; funding acquisition, YL All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
The study was conducted according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Ethics Committee for Animal Experimentation of Chongqing Medical University (number SCXK (Yu) 2018–0003). All efforts were made to minimize the number of rats sacrificed and their suffering.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, Y., Jiang, J., Jiang, W. et al. Spata2 Knockdown Exacerbates Brain Inflammation via NF-κB/P38MAPK Signaling and NLRP3 Inflammasome Activation in Cerebral Ischemia/Reperfusion Rats. Neurochem Res 46, 2262–2275 (2021). https://doi.org/10.1007/s11064-021-03360-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03360-8