Abstract
Parkinson’s disease (PD) is a slow progressive, second most common neurodegenerative disease characterized by the loss of dopaminergic neurons from the nigrostriatal pathway. In spite of extensive research the therapeutics options of disease are limited which only offer symptomatic relief and could not prevent the disease progression. Therefore researchers are looking for the probable synthetic or natural compounds for the PD therapeutics. Due to mandatory chronic consumption of anti PD drug to the PD patients, the natural compounds are getting attention recently. Numerous studies have indicated the neuroprotective effects of natural polyphenols including epigallocatechin, quercetin, baicalein, resveratrol, luteolin, curcumin, puerarin, genistein, hyperoside naringin against dopaminergic neuronal death with relatively safe with uncommon, mild or transient side effects. However, their mechanistic interference in dopaminergic neuronal death mechanism is not very well defined. Herein, we have attempted to discuss the various natural polyphenols with their known effects on various PD related pathologies to understand their therapeutic utilization for PD patients either in prophylactic or therapeutic mode. Briefly we have also discussed the major disease mechanisms which could be targeted for utilization of these polyphenols specifically involving oxidative stress and mitochondrial dysfunction. We have also discuss the limitation and probable strategies for the clinical utilization of these polyphenols for the benefit of PD patients.
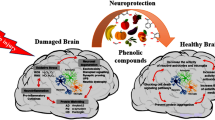
Graphic Abstract

Similar content being viewed by others
References
DeMaagd G, Philip A (2015) Parkinson’s disease and its management part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm Ther 40:504–532
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Singh S, Dikshit M (2007) Apoptotic neuronal death in Parkinson’s disease: involvement of nitric oxide. Brain Res Rev 54:233–250. https://doi.org/10.1016/j.brainresrev.2007.02.001
Singh S, Kumar S, Dikshit M (2010) Involvement of the mitochondrial apoptotic pathway and nitric oxide synthase in dopaminergic neuronal death induced by 6-hydroxydopamine and lipopolysaccharide. Redox Rep 15:115–122. https://doi.org/10.1179/174329210X12650506623447
Li J, Wuliji O, Li W, Jiang ZG, Ghanbari HA (2013) Oxidative stress and neurodegenerative disorders. Int J Mol Sci 14:24438–24475. https://doi.org/10.3390/ijms141224438
Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G (2016) The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. https://doi.org/10.1155/2016/9730467
Cannon JR, Greenamyre JT (2011) The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 124:225–250. https://doi.org/10.1093/toxsci/kfr239
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in parkinson’s disease. J Parkinsons Dis 3:461–491. https://doi.org/10.3233/JPD-130230
Francini A, Sebastiani L (2013) Phenolic compounds in apple (Malus x domestica borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2:181–193. https://doi.org/10.3390/antiox2030181
Garbarino VR, Orr ME, Rodriguez KA, Buffenstein R (2015) Mechanisms of oxidative stress resistance in the brain: lessons learned from hypoxia tolerant extremophilic vertebrates. Arch Biochem Biophys 576:8–16. https://doi.org/10.1016/j.abb.2015.01.029
Shrikanta A, Kumar A, Govindaswamy V (2015) Resveratrol content and antioxidant properties of underutilized fruits. J Food Sci Technol 52:383–390. https://doi.org/10.1007/s13197-013-0993-z
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344. https://doi.org/10.1113/jphysiol.2003.049478
Drechsel DA, Patel M (2008) Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med 44:1873–1886. https://doi.org/10.1016/j.freeradbiomed.2008.02.008
Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP (2013) Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 236:136–148. https://doi.org/10.1016/j.neuroscience.2013.01.032
Murphy MP (2014) Antioxidants as therapies: can we improve on nature? Free Radic Biol Med 66:20–23. https://doi.org/10.1016/j.freeradbiomed.2013.04.010
Pervaiz T, Songtao J, Faghihi F, Haider MS, Fang J (2017) Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J Plant Biochem Physiol. https://doi.org/10.4172/2329-9029.1000187
Aprioku JS (2013) Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil 14:158–172
Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL (2004) The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J Biol Chem 279:26846–26857. https://doi.org/10.1074/jbc.M403129200
Song N, Jiang H, Wang J, Xie JX (2007) Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 85:3118–3126. https://doi.org/10.1002/jnr.21430
Xu H, Jiang H, Wang J, Xie J (2010) Rg1 protects iron-induced neurotoxicity through antioxidant and iron regulatory proteins in 6-OHDA-treated MES23.5 cells. J Cell Biochem 111:1537–1545. https://doi.org/10.1002/jcb.22885
Dawson TM, Dawson VL, Snyder SH (1992) A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol 32:297–311. https://doi.org/10.1002/ana.410320302
Singh S, Das T, Ravindran A, Chaturvedi RK, Shukla Y, Agarwal AK, Dikshit M (2005) Involvement of nitric oxide in neurodegeneration: a study on the experimental models of Parkinson’s disease. Redox Rep 10:103–109. https://doi.org/10.1179/135100005X38842
Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA, Darley-Usmar VM (1999) Biological aspects of reactive nitrogen species. Biochim Biophys Acta 1411:385–400. https://doi.org/10.1016/s0005-2728(99)00028-6
Lushchak VI (2012) Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J Amino Acids 2012:1–26. https://doi.org/10.1155/2012/736837
Hu Q, Wang G (2016) Mitochondrial dysfunction in Parkinson’s disease. Transl Neurodegener. https://doi.org/10.1186/s40035-016-0060-6
Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A (2006) Mitochondrial complex I: structural and functional aspects. Biochim Biophys Acta - Bioenerg 1757:1406–1420. https://doi.org/10.1016/j.bbabio.2006.05.007
Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827. https://doi.org/10.1111/j.1471-4159.1990.tb02325.x
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta - Mol Basis Dis 1802:29–44. https://doi.org/10.1016/j.bbadis.2009.08.013
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494. https://doi.org/10.1602/neurorx.2.3.484
Aon MA, Cortassa S, Maack C, O’Rourke B (2007) Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282:21889–21900. https://doi.org/10.1074/jbc.M702841200
Kalani K, Yan SF, Du Yan SS (2018) Mitochondrial permeability transition pore: a potential drug target for neurodegeneration. Drug Discov Today 23:1983–1989. https://doi.org/10.1016/j.drudis.2018.08.001
George J, Mok S, Moses D, Wilkins S, Bush A, Cherny R, Finkelstein D (2009) Targeting the progression of Parkinsons Disease. Curr Neuropharmacol 7:9–36. https://doi.org/10.2174/157015909787602814
Knight JA (2020) Reactive oxygen species and the neurodegenerative disorders. Ann Clin Lab Sci 27(1):11–25
Wattanathorn J, Sriraksa N, Muchimapura S, Tiamkao S, Brown K, Chaisiwamongkol K (2012) Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid-Based Complement Altern Med. https://doi.org/10.1155/2012/823206
Weinreb O, Amit T, Mandel S, Youdim MBH (2009) Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr 4:283–296. https://doi.org/10.1007/s12263-009-0143-4
Vaughan RA, Foster JD (2013) Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 34:489–496. https://doi.org/10.1016/j.tips.2013.07.005
Shamoto-Nagai M, Maruyama W, Yi H, Akao Y, Tribl F, Gerlach M, Osawa T, Riederer P, Naoi M (2006) Neuromelanin induces oxidative stress in mitochondria through release of iron: mechanism behind the inhibition of 26S proteasome. J Neural Transm 113:633–644. https://doi.org/10.1007/s00702-005-0410-5
Lohr KM, Miller GW (2014) VMAT2 and Parkinson’s disease: harnessing the dopamine vesicle. Expert Rev Neurother 14(10):1115–1117
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat. https://doi.org/10.3389/fnana.2015.00091
Skouta R (2016) Neuroprotective effect of antioxidant compounds. Neural Regen Res 11:566. https://doi.org/10.4103/1673-5374.180738
Pohl F, Lin PKT (2018) The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: in vitro, in vivo and clinical trials. Molecules. https://doi.org/10.3390/molecules23123283
Apostolova N, Victor VM (2015) Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal 22:686–729. https://doi.org/10.1089/ars.2014.5952
Kujawska M, Jodynis-Liebert J (2018) Polyphenols in Parkinson’s disease: a systematic review of in vivo studies. Nutrients 10:642. https://doi.org/10.3390/nu10050642
He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z (2015) Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 20:9183–9213. https://doi.org/10.3390/molecules20059183
Uttara B, Singh A, Zamboni P, Mahajan R (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74. https://doi.org/10.2174/157015909787602823
Jin F, Wu Q, Lu YF, Gong QH, Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol 600:78–82. https://doi.org/10.1016/j.ejphar.2008.10.005
Henchcliffe C, Beal FM (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609. https://doi.org/10.1038/ncpneuro0924
Young AJ, Johnson S, Steffens DC, Doraiswamy PM (2007) Coenzyme Q10: a review of its promise as a neuroprotectant. CNS Spectr 12:62–68. https://doi.org/10.1017/s1092852900020538
Shults CW, Haas RH, Beal MF (1999) A possible role of coenzyme Q10 in the etiology and treatment of Parkinson’s disease. BioFactors 9:267–272. https://doi.org/10.1002/biof.5520090223
Zhou YX, Zhang H, Peng C (2014) Puerarin: a review of pharmacological effects. Phyther Res 28:961–975. https://doi.org/10.1002/ptr.5083
Bhullar KS, Rupasinghe HPV (2013) Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev. https://doi.org/10.1155/2013/891748
Costa LG, Garrick JM, Roquè PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2986796
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med. https://doi.org/10.1186/1749-8546-5-13
Elufioye TO, Berida TI, Habtemariam S (2017) Plants-derived neuroprotective agents: cutting the cycle of cell death through multiple mechanisms. Evid-Based Complement Altern Med. https://doi.org/10.1155/2017/3574012
Chu C, Deng J, Man Y, Qu Y (2017) Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed Res Int. https://doi.org/10.1155/2017/5615647
Chan MMY, Fong D, Ho CT, Huang HI (1997) Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem Pharmacol 54:1281–1286. https://doi.org/10.1016/S0006-2952(97)00504-2
Li FJ, Ji HF, Shen L (2012) A meta-analysis of tea drinking and risk of Parkinson’s disease. Sci World J. https://doi.org/10.1100/2012/923464
Perez C, Tong Y, Guo M (2008) Iron chelators as potential therapeutic agents for Parkinsons disease. Curr Bioact Compd 4:150–158. https://doi.org/10.2174/157340708786305952
Guo S, Yan J, Yang T, Yang X, Bezard E, Zhao B (2007) Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol Psychiatry 62:1353–1362. https://doi.org/10.1016/j.biopsych.2007.04.020
Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE (2010) EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA 107:7710–7715. https://doi.org/10.1073/pnas.0910723107
Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J (2015) The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett 589:77–83. https://doi.org/10.1016/j.febslet.2014.11.026
Muangpaisan W, Hori H, Brayne C (2009) Systematic review of the prevalence and incidence of Parkinson’s disease in Asia. J Epidemiol 19:281–293. https://doi.org/10.2188/jea.je20081034
Muangpaisan W, Mathews A, Hori H, Seidel D (2011) A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J Med Assoc Thail 94:749–755
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10:84–89. https://doi.org/10.4103/0973-7847.194044
Haleagrahara N, Siew CJ, Mitra NK, Kumari M (2011) Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci Lett 500:139–143. https://doi.org/10.1016/j.neulet.2011.06.021
Bakoyiannis I, Daskalopoulou A, Pergialiotis V, Perrea D (2019) Phytochemicals and cognitive health: are flavonoids doing the trick? Biomed Pharmacother 109:1488–1497. https://doi.org/10.1016/j.biopha.2018.10.086
Haleagrahara N, Siew CJ, Ponnusamy K (2013) Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-OHDA) induced neurotoxicity in striatum of rats. J Toxicol Sci 38:25–33. https://doi.org/10.2131/jts.38.25
Zhang ZJ, Cheang LCV, Wang MW, Lee SMY (2011) Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int J Mol Med 27:195–203. https://doi.org/10.3892/ijmm.2010.571
Kelly GS (2011) Quercetin. Monograph. Altern Med Rev 16:172–9409
Goldberg DM, Yan J, Soleas GJ (2003) Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem 36:79–87. https://doi.org/10.1016/S0009-9120(02)00397-1
Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S (2017) Baicalein as a potent neuroprotective agent: a review. Biomed Pharmacother 95:1021–1032. https://doi.org/10.1016/j.biopha.2017.08.135
Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S (2018) Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sci. https://doi.org/10.3390/brainsci8060104
Chen SF, Hsu CW, Huang WH, Wang JY (2008) Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol 155:1279–1296. https://doi.org/10.1038/bjp.2008.345
Yu X, He GR, Sun L, Lan X, Shi LL, Xuan ZH, Du GH (2012) Assessment of the treatment effect of baicalein on a model of Parkinsonian tremor and elucidation of the mechanism. Life Sci 91:5–13. https://doi.org/10.1016/j.lfs.2012.05.005
Kanthasamy K, Gordon R, Jin H, Anantharam V, Ali S, Kanthasamy AG, Kanthasamy A (2011) Neuroprotective effect of resveratrol against methamphetamine-induced dopaminergic apoptotic cell death in a cell culture model of neurotoxicity. Curr Neuropharmacol 9:49–53. https://doi.org/10.2174/157015911795017353
Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A (2016) The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci. https://doi.org/10.3390/ijms17020160
Athauda D, Foltynie T (2015) The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol 11:25–40. https://doi.org/10.1038/nrneurol.2014.226
Wang ZH, Zhang JL, Duan YL, Zhang QS, Li GF, Zheng DL (2015) MicroRNA-214 participates in the neuroprotective effect of resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomed Pharmacother 74:252–256. https://doi.org/10.1016/j.biopha.2015.08.025
Chang CY, Choi DK, Lee DK, Hong YJ, Park EJ (2013) Resveratrol confers protection against rotenone-induced neurotoxicity by modulating myeloperoxidase levels in glial cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0060654
Asif M, Khodadadi E (2013) Medicinal uses and chemistry of flavonoid contents of some common edible tropical plants. J Paramed Sci 4:119–138. https://doi.org/10.22037/jps.v4i3.4648
Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW (2008) Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci Lett 448:175–179. https://doi.org/10.1016/j.neulet.2008.10.046
Hu LW, Yen JH, Shen YT, Wu KY, Wu MJ (2014) Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0097880
Yan T, Li L, Sun B, Liu F, Yang P, Chen T, Li T, Liu X (2014) Luteolin inhibits behavioral sensitization by blocking methamphetamine- induced MAPK pathway activation in the caudate putamen in mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0098981
Zhao G, Yao-Yue C, Qin GW, Guo LH (2012) Luteolin from purple perilla mitigates ROS insult particularly in primary neurons. Neurobiol Aging 33:176–186. https://doi.org/10.1016/j.neurobiolaging.2010.02.013
Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11:338–378. https://doi.org/10.2174/1570159X11311040002
Liu Z, Yu Y, Li X, Ross CA, Smith WW (2011) Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for parkinsonism. Pharmacol Res 63:439–444. https://doi.org/10.1016/j.phrs.2011.01.004
Lee HS, Jung KK, Cho JY, Rhee MH, Hong S, Kwon M, Kim SH, Kang SY (2007) Neuroprotective effect of curcumin is mainly mediated by blockade of microglial cell activation. Pharmazie 62:937–942
Zhu G, Wang X, Wu S, Li Q (2012) Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP +-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int 60:400–408. https://doi.org/10.1016/j.neuint.2012.01.003
Wang G, Zhou L, Zhang Y, Dong M, Li X, Liu J, Niu Y (2011) Implication of the c-Jun-NH2-terminal kinase pathway in the neuroprotective effect of puerarin against 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12 cells. Neurosci Lett 487:88–93. https://doi.org/10.1016/j.neulet.2010.10.002
Zhang X, Xiong J, Liu S, Wang L, Huang J, Liu L, Yang J, Zhang G, Guo K, Zhang Z, Wu P, Wang D, Lin Z, Xiong N, Wang T (2014) Puerarin protects dopaminergic neurons in Parkinson’s disease models. Neuroscience 280:88–98. https://doi.org/10.1016/j.neuroscience.2014.08.052
Zárate S, Stevnsner T, Gredilla R (2017) Role of estrogen and other sex hormones in brain aging. Neuroprotection and DNA repair. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2017.00430
Liu LX, Chen WF, Xie JX, Wong MS (2008) Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci Res 60:156–161. https://doi.org/10.1016/j.neures.2007.10.005
Lin CM, Lin RD, Chen ST, Lin YP, Chiu WT, Lin JW, Hsu FL, Lee MH (2010) Neurocytoprotective effects of the bioactive constituents of Pueraria thomsonii in 6-hydroxydopamine (6-OHDA)-treated nerve growth factor (NGF)-differentiated PC12 cells. Phytochemistry 71:2147–2156. https://doi.org/10.1016/j.phytochem.2010.08.015
Nielsen ILF, Williamson G (2007) Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer 57:1–10. https://doi.org/10.1080/01635580701267677
Jiang F, Gao R, Liu H, Zhao D, Xu P, Zhang L, Qian X (2016) Neuroprotective effect of hyperoside on human PC12 cells against the oxidative damage. Int J Clin Exp Pathol 9:5176–5183
Gopinath K, Prakash D, Sudhandiran G (2011) Neuroprotective effect of naringin, a dietary flavonoid against 3-Nitropropionic acid-induced neuronal apoptosis. Neurochem Int 59:1066–1073. https://doi.org/10.1016/j.neuint.2011.08.022
Leem E, Nam JH, Jeon MT, Shin WH, Won SY, Park SJ, Choi MS, Jin BK, Jung UJ, Kim SR (2014) Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of parkinson’s disease. J Nutr Biochem 25:801–806. https://doi.org/10.1016/j.jnutbio.2014.03.006
Hui W, Xu YS, Miao Lin W, Chao C, Bian R, Yuan H, Yi W, Guo T, Zhu LL, Zhou H (2017) Protective effect of naringin against the LPS-induced apoptosis of PC12 cells: Implications for the treatment of neurodegenerative disorders. Int J Mol Med 39:819–830. https://doi.org/10.3892/ijmm.2017.2904
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39:1119–1125. https://doi.org/10.1080/10715760500233113
Jung UJ, Kim SR (2014) Effects of naringin, A flavanone glycoside in grapefruits and citrus fruits, On the nigrostriatal dopaminergic projection in the adult brain, Neural Regen. Res 9:1514–1517. https://doi.org/10.4103/1673-5374.139476
Renaud J, Martinoli MG (2019) Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int J Mol Sci 20(8):1883. https://doi.org/10.3390/ijms20081883
Huang W, Lee SL, Yu LX (2009) Mechanistic approaches to predicting oral drug absorption. AAPS J 11(2):217–224. https://doi.org/10.1208/s12248-009-9098-z
Sawai Y, Kohsaka K, Nishiyama Y, Ando K (1987) Serum concentrations of rutoside metabolites after oral administration of a rutoside formulation to humans. Arzneim-Forsch 37(6):729–732
Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila SS, Rice-Evans C, Spencer JP (2006) Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br J Nutr 96(1):62–70. https://doi.org/10.1079/bjn20061810
Doostdar H, Burke MD, Mayer RT (2000) Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology 144(1–3):31–38. https://doi.org/10.1016/s0300-483x(99)00215-2
Riva S, Monti D, Luisetti M, Danieli B (1998) Enzymatic modification of natural compounds with pharmacological properties. Ann N Y Acad Sci 864:70–80. https://doi.org/10.1111/j.1749-6632.1998.tb10289.x
Chao J, Li H, Cheng KW, Yu MS, Chang RC, Wang M (2010) Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J Nutr Biochem 21(6):482–489. https://doi.org/10.1016/j.jnutbio.2009.02.004
Souto EB, Severino P, Basso R, Santana MH (2013) Encapsulation of antioxidants in gastrointestinal-resistant nanoparticulate carriers. Methods Mol Biol (Clifton, N.J.) 1028:37–46. https://doi.org/10.1007/978-1-62703-475-3_3
Fang Z, Bhandari B (2010) Encapsulation of polyphenols—a review. Trends Food Sci Technol 21:510–523
Dewey RB, Maraganore Jr DM, Ahlskog JE, Matsumoto JY (1998) A double-blind, placebo-controlled study of intranasal apomorphine spray as a rescue agent for off-states in Parkinson's disease. Mov Disord 13(5):782–787. https://doi.org/10.1002/mds.870130505
Hanson LR, Frey WH II (2008) Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 9(Suppl 3):S5. https://doi.org/10.1186/1471-2202-9-S3-S5
Tseng HC, Wang MH, Chang KC, Soung HS, Fang CH, Lin YW, Li KY, Yang CC, Tsai CC (2020) Protective effect of (-)epigallocatechin-3-gallate on rotenone-induced Parkinsonism-like symptoms in rats. Neurotox Res 37(3):669–682
Xu Q, Langley M, Kanthasamy AG, Reddy MB (2017) Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J Nutr 147(10):1926–1931. https://doi.org/10.3945/jn.117.255034
Chen D, Kanthasamy AG, Reddy MB (2015) EGCG protects against 6-OHDA-induced neurotoxicity in a cell culture model. Parkinson's Dis 2015:843906. https://doi.org/10.1155/2015/843906
Bitu Pinto N, da Silva Alexandre B, Neves KR, Silva AH, Leal LK, Viana GS (2015) Neuroprotective properties of the standardized extract from Camellia sinensis (Green Tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of Parkinson's disease. Evid-Based Complement Altern Med 2015:161092. https://doi.org/10.1155/2015/161092
Ghaffari F, Hajizadeh Moghaddam A, Zare M (2018) Neuroprotective effect of quercetin nanocrystal in a 6-hydroxydopamine model of Parkinson disease: biochemical and behavioral evidence. Basic Clin Neurosci 9(5):317–324. https://doi.org/10.32598/bcn.9.5.317
El-Horany HE, El-Latif RN, El Batsh MM, Emam MN (2016) Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of parkinson's disease: modulating autophagy (quercetin on experimental Parkinson's disease). J Biochem Mol Toxicol 30(7):360–369. https://doi.org/10.1002/jbt.21821
Sharma DR, Wani WY, Sunkaria A, Kandimalla RJ, Sharma RK, Verma D, Bal A, Gill KD (2016) Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 324:163–176. https://doi.org/10.1016/j.neuroscience.2016.02.055
Denny Joseph KM, Muralidhara, (2015) Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: relevance to Parkinson's disease. Neurochem Res 40(5):894–905. https://doi.org/10.1007/s11064-015-1542-0
Zheng ZV, Cheung CY, Lyu H, Chan HY, Li Y, Bian ZX, Wang K, Poon WS (2019) Baicalein enhances the effect of low dose Levodopa on the gait deficits and protects dopaminergic neurons in experimental Parkinsonism. J Clin Neurosci 64:242–251. https://doi.org/10.1016/j.jocn.2019.02.005
Zhang X, Yang Y, Du L, Zhang W, Du G (2017) Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int Immunopharmacol 50:38–47. https://doi.org/10.1016/j.intimp.2017.06.007
Wang SF, Liu LF, Wu MY, Cai CZ, Su H, Tan J, Lu JH, Li M (2017) Baicalein prevents 6-OHDA/ascorbic acid-induced calcium-dependent dopaminergic neuronal cell death. Sci Rep 7(1):8398. https://doi.org/10.1038/s41598-017-07142-7
Kuang L, Cao X, Lu Z (2017) Baicalein protects against rotenone-induced neurotoxicity through induction of autophagy. Biol Pharm Bull 40(9):1537–1543. https://doi.org/10.1248/bpb.b17-00392
Zhang X, Du L, Zhang W, Yang Y, Zhou Q, Du G (2017) Therapeutic effects of baicalein on rotenone-induced Parkinson's disease through protecting mitochondrial function and biogenesis. Sci Rep 7(1):9968. https://doi.org/10.1038/s41598-017-07442-y
Xia D, Sui R, Zhang Z (2019) Administration of resveratrol improved Parkinson's disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J Cell Biochem 120(4):4942–4951. https://doi.org/10.1002/jcb.27769
Lin KL, Lin KJ, Wang PW, Chuang JH, Lin HY, Chen SD, Chuang YC, Huang ST, Tiao MM, Chen JB, Huang PH, Liou CW, Lin TK (2018) Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic Res 52(11–12):1371–1386. https://doi.org/10.1080/10715762.2018.1489128
Palle S, Neerati P (2018) Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson's disease. Naunyn-Schmiedeberg's Arch Pharmacol 391(4):445–453. https://doi.org/10.1007/s00210-018-1474-8
Wu Z, Wu A, Dong J, Sigears A, Lu B (2018) Grape skin extract improves muscle function and extends lifespan of a Drosophila model of Parkinson's disease through activation of mitophagy. Exp Gerontol 113:10–17. https://doi.org/10.1016/j.exger.2018.09.014
Zhang LF, Ji XL, Yu M, Liu SY, Wu XL, Wang YJ, Liu RT (2018) Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson's disease. Food Funct 9(12):6414–6426. https://doi.org/10.1039/c8fo00964c
Gaballah HH, Zakaria SS, Elbatsh MM, Tahoon NM (2016) Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson's disease. Chem-Biol Interact 251:10–16. https://doi.org/10.1016/j.cbi.2016.03.023
Qin L, Chen Z, Yang L, Shi H, Wu H, Zhang B, Zhang W, Xu Q, Huang F, Wu X (2019) Luteolin-7-O-glucoside protects dopaminergic neurons by activating estrogen-receptor-mediated signaling pathway in MPTP-induced mice. Toxicology 426:152256. https://doi.org/10.1016/j.tox.2019.152256
Elmazoglu Z, Yar Saglam AS, Sonmez C, Karasu C (2020) Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson's disease and inflammatory pathways. Drug Chem Toxicol 43(1):96–103. https://doi.org/10.1080/01480545.2018.1504961
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang W, Sun Y, Zhang H (2018) Curcumin protects an SH-SY5Y cell model of Parkinson's disease against toxic injury by regulating HSP90. Cell Physiol Biochem 51(2):681–691. https://doi.org/10.1159/000495326
Sharma N, Nehru B (2018) Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson's disease model. Inflammopharmacology 26(2):349–360. https://doi.org/10.1007/s10787-017-0402-8
Darbinyan LV, Hambardzumyan LE, Simonyan KV, Chavushyan VA, Manukyan LP, Badalyan SA, Khalaji N, Sarkisian VH (2017) Protective effects of curcumin against rotenone-induced rat model of Parkinson's disease: in vivo electrophysiological and behavioral study. Metab Brain Dis 32(6):1791–1803. https://doi.org/10.1007/s11011-017-0060-y
van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, Bardien S (2017) Curcumin rescues a PINK1 knock down SH-SY5Y cellular model of Parkinson's disease from mitochondrial dysfunction and cell death. Mol Neurobiol 54(4):2752–2762. https://doi.org/10.1007/s12035-016-9843-0
Shiying L, Xinhui Q, Guanghua J, Feng N, Feng L, Shumei C, Fan H (2018) Puerarin promoted proliferation and differentiation of dopamine-producing cells in Parkinson's animal models. Biomed Pharmacother Biomed Pharm 106:1236–1242. https://doi.org/10.1016/j.biopha.2018.07.058
Cheng YF, Zhu G, Wu QW, Xie YS, Jiang Y, Guo L, Guan YL, Liu YS, Zhang J (2017) GPR30 activation contributes to the puerarin-mediated neuroprotection in MPP+-induced SH-SY5Y cell death. J Mol Neurosci 61(2):227–234. https://doi.org/10.1007/s12031-016-0856-y
Wu HC, Hu QL, Zhang SJ, Wang YM, Jin ZK, Lv LF, Zhang S, Liu ZL, Wu HL, Cheng OM (2018) Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen Res 13(8):1375–1383. https://doi.org/10.4103/1673-5374.235250
Siddique YH, Naz F, Jyoti S, Ali F, Rahul, (2019) Effect of genistein on the transgenic drosophila model of Parkinson's disease. J Diet Suppl 16(5):550–563. https://doi.org/10.1080/19390211.2018.1472706
Fan HH, Zhu LB, Li T, Zhu H, Wang YN, Ren XL, Hu BL, Huang CP, Zhu JH, Zhang X (2017) Hyperoside inhibits lipopolysaccharide-induced inflammatory responses in microglial cells via p38 and NFκB pathways. Int Immunopharmacol 50:14–21. https://doi.org/10.1016/j.intimp.2017.06.004
Xing HY, Cai YQ, Wang XF, Wang LL, Li P, Wang GY, Chen JH (2015) The cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS ONE 10(12):e0145183. https://doi.org/10.1371/journal.pone.0145183
Liu Z, Tao X, Zhang C, Lu Y, Wei D (2005) Protective effects of hyperoside (quercetin-3-o-galactoside) to PC12 cells against cytotoxicity induced by hydrogen peroxide and tert-butyl hydroperoxide. Biomed Pharm 59(9):481–490. https://doi.org/10.1016/j.biopha.2005.06.009
Garabadu D, Agrawal N (2020) Naringin exhibits neuroprotection against rotenone-induced neurotoxicity in experimental rodents. NeuroMol Med. https://doi.org/10.1007/s12017-019-08590-2
Kim HD, Jeong KH, Jung UJ, Kim SR (2016) Naringin treatment induces neuroprotective effects in a mouse model of Parkinson's disease in vivo, but not enough to restore the lesioned dopaminergic system. J Nutr Biochem 28:140–146. https://doi.org/10.1016/j.jnutbio.2015.10.013
Jung UJ, Leem E, Kim SR (2014) Naringin: a protector of the nigrostriatal dopaminergic projection. Exp Neurobiol 23(2):124–129. https://doi.org/10.5607/en.2014.23.2.124
Acknowledgements
Authors acknowledge Science and Engineering Research Board (PDF/2017/000984) and Indian Council of Medical Research (2016-0624/CMB/Adhoc-BMS) for financial support.
Author information
Authors and Affiliations
Contributions
Ashish Singh wrote and designed the manuscript, Pratibha Tripathi and Arun Kumar Yadawa wrote the manuscript. Sarika Singh designed and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This manuscript does not involve human or animal use.
Informed Consent
There is no requirement of consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Tripathi, P., Yadawa, A.K. et al. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem Res 45, 1731–1745 (2020). https://doi.org/10.1007/s11064-020-03058-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03058-3