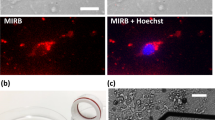
Abstract
Due to their exciting properties, engineered nanoparticles have obtained substantial attention over the last two decades. As many types of nanoparticles are already used for technical and biomedical applications, the chances that cells in the brain will encounter nanoparticles have strongly increased. To test for potential consequences of an exposure of brain cells to engineered nanoparticles, cell culture models for different types of neural cells are frequently used. In this review article we will discuss experimental strategies and important controls that should be used to investigate the physicochemical properties of nanoparticles for the cell incubation conditions applied as well as for studies on the biocompatibility and the cellular uptake of nanoparticles in neural cells. The main focus of this article will be the interaction of cultured neural cells with iron oxide nanoparticles, but similar considerations are important for studying the consequences of an exposure of other types of cultured cells with other types of nanoparticles. Our article aims to improve the understanding of the special technical challenges of working with nanoparticles on cultured neural cells, to identify potential artifacts and to prevent misinterpretation of data on the potential adverse or beneficial consequences of a treatment of cultured cells with nanoparticles.



Similar content being viewed by others
References
Mohammed L, Gomaa HG, Ragab D, Zhu J (2017) Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology 30:1–14
Patil US, Adireddy S, Jaiswal A, Mandava S, Lee BR, Chrisey DB (2015) In vitro/in vivo toxicity evaluation and quantification of iron oxide nanoparticles. Int J Mol Sci 16:24417–24450
Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 4:634–641
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR (2016) Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33:2373–2387
Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB, Heverhagen JT, Prosperi D, Parak WJ (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41:4306–4334
De la Fuente JM, Grazu V (2012) Nanobiotechnology: inorganic nanoparticles vs organic nanoparticles. Elsevier, Oxford
Algar WR, Prasuhn DE, Stewart MH, Jennings TL, Blanco-Canosa JB, Dawson PE, Medintz IL (2011) The controlled display of biomolecules on nanoparticles: a challenge suited to bioorthogonal chemistry. Bioconjug Chem 22:825–858
Feliu N, Docter D, Heine M, Del Pino P, Ashraf S, Kolosnjaj-Tabi J, Macchiarini P, Nielsen P, Alloyeau D, Gazeau F, Stauber RH, Parak WJ (2016) In vivo degeneration and the fate of inorganic nanoparticles. Chem Soc Rev 45:2440–2457
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Valdiglesias V, Kilic G, Costa C, Fernandez-Bertolez N, Pasaro E, Teixeira JP, Laffon B (2015) Effects of iron oxide nanoparticles: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ Mol Mutagen 56:125–148
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021
Ali A, Zafar H, Zia M, Ul Haq I, Phull AR, Ali JS, Hussain A (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 9:49–67
Sperling RA, Parak WJ (2010) Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans A 368:1333–1383
Oh N, Park JH (2014) Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomed 9:51–63
McNamara K, Tofail SAM (2017) Nanoparticles in biomedical applications. Adv Phys X 2:54–88
Nazarenus M, Zhang Q, Soliman MG, Del Pino P, Pelaz B, Carregal-Romero S, Rejman J, Rothen-Rutishauser B, Clift MJ, Zellner R, Nienhaus GU, Delehanty JB, Medintz IL, Parak WJ (2014) In vitro interaction of colloidal nanoparticles with mammalian cells: what have we learned thus far? Beilstein J Nanotechnol 5:1477–1490
Rivera-Gil P, Jimenez de Aberasturi D, Wulf V, Pelaz B, del Pino P, Zhao Y, de la Fuente JM, Ruiz de Larramendi I, Rojo T, Liang XJ, Parak WJ (2013) The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc Chem Res 46:743–749
Costa C, Brandao F, Bessa MJ, Costa S, Valdiglesias V, Kilic G, Fernandez-Bertolez N, Quaresma P, Pereira E, Pasaro E, Laffon B, Teixeira JP (2016) In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J Appl Toxicol 36:361–372
Petters C, Irrsack E, Koch M, Dringen R (2014) Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem Res 39:1648–1660
Meindl C, Oehlinger K, Ober J, Roblegg E, Froehlich E (2017) Comparison of fluorescence-based methods to determine nanoparticle uptake by phagocytes and non-phagocytic cells in vitro. Toxicology 378:25–36
Soenen SJ, Parak WJ, Rejman J, Manshian B (2015) (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev 115:2109–2135
Kura AU, Fakurazi S, Hussein MZ, Arulselvan P (2014) Nanotechnology in drug delivery: the need for more cell culture based studies in screening. Chem Cent J 8:46
Calero M, Gutierrez L, Salas G, Luengo Y, Lazaro A, Acedo P, Morales MP, Miranda R, Villanueva A (2014) Efficient and safe internalization of magnetic iron oxide nanoparticles: two fundamental requirements for biomedical applications. Nanomedicine 10:733–743
Valdiglesias V, Fernandez-Bertolez N, Kilic G, Costa C, Costa S, Fraga S, Bessa MJ, Pasaro E, Teixeira JP, Laffon B (2016) Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J Trace Elem Med Biol 38:53–63
Worle-Knirsch JM, Pulskamp K, Krug HF (2006) Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett 6:1261–1268
Bregoli L, Benetti F, Venturini M, Sabbioni E (2013) ECSIN’s methodological approach for hazard evaluation of engineered nanomaterials. J Phys Conf Ser 429:012017
Corr SA, Rakovich YP, Gun’ko YK (2008) Multifunctional magnetic-fluorescent nanocomposites for biomedical applications. Nanoscale Res Lett 3:87–104
Weinstein JS, Varallyay CG, Dosa E, Gahramanov S, Hamilton B, Rooney WD, Muldoon LL, Neuwelt EA (2010) Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 30:15–35
Wang YX (2015) Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J Gastroenterol 21:13400–13402
Baetke SC, Lammers T, Kiessling F (2015) Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol 88:1054
Shi D, Sadat ME, Dunn AW, Mast DB (2015) Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale 7:8209–8232
Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, Pottler M, Durr S, Alexiou C (2015) Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun 468:463–470
El-Boubbou K (2018) Magnetic iron oxide nanoparticles as drug carriers: clinical relevance. Nanomedicine. https://doi.org/10.2217/nnm-2017-0336
Ivask A, Pilkington EH, Blin T, Kakinen A, Vija H, Visnapuu M, Quinn JF, Whittaker MR, Qiao R, Davis TP, Ke PC, Voelcker NH (2018) Uptake and transcytosis of functionalized superparamagnetic iron oxide nanoparticles in an in vitro blood brain barrier model. Biomater Sci 6:314–323
Yang H (2010) Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res 27:1759–1771
Kaewsaneha C, Tangboriboonrat P, Polpanich D, Elaissari A (2015) Multifunctional fluorescent-magnetic polymeric colloidal particles: preparations and bioanalytical applications. ACS Appl Mater Interfaces 7:23373–23386
Shi D, Mi G, Bhattacharya S, Nayar S, Webster TJ (2016) Optimizing superparamagnetic iron oxide nanoparticles as drug carriers using an in vitro blood-brain barrier model. Int J Nanomed 11:5371–5379
Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK (2018) Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res 341:154–175
Hare D, Ayton S, Bush A, Lei P (2013) A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 5:1–19
Rouault TA (2013) Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci 14:551–564
Hohnholt MC, Geppert M, Luther EM, Petters C, Bulcke F, Dringen R (2013) Handling of iron oxide and silver nanoparticles by astrocytes. Neurochem Res 38:227–239
Bencsik A, Lestaevel P, Guseva Canu I (2018) Nano- and neurotoxicology: an emerging discipline. Prog Neurobiol 160:45–63
Reynolds JL, Mahato RI (2017) Nanomedicines for the treatment of CNS diseases. J Neuroimmune Pharmacol 12:1–5
Zhou Y, Peng Z, Seven ES, Leblanc RM (2018) Crossing the blood-brain barrier with nanoparticles. J Control Release 270:290–303
Das S, Carnicer-Lombarte A, Fawcett JW, Bora U (2016) Bio-inspired nano tools for neuroscience. Prog Neurobiol 142:1–22
Mahmoudi M, Meng J, Xue X, Liang XJ, Rahman M, Pfeiffer C, Hartmann R, Gil PR, Pelaz B, Parak WJ, Del Pino P, Carregal-Romero S, Kanaras AG, Tamil Selvan S (2014) Interaction of stable colloidal nanoparticles with cellular membranes. Biotechnol Adv 32:679–692
Shang L, Nienhaus K, Nienhaus GU (2014) Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol 12:1–11
Petri-Fink A, Steitz B, Finka A, Salaklang J, Hofmann H (2008) Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studies. Eur J Pharm Biopharm 68:129–137
Kumar A, Dixit CK (2017) Methods for characterization of nanoparticles. In: Nimesh S, Ramesh C, Gupta N (eds) Advances in nanomedicine for the delivery of therapeutic nucleic acids. Woodhead Publishing, Cambridge, pp 43–58
Bhatia S (2016) Nanoparticles types, classification, characterization, fabrication methods and drug dilvery. In: Bhatia S (ed) Natural polymer drug delivery systems: nanoparticle, plats, and algae, 1 edn. Springer, Cham, pp 33–93
Wang R, Mikoryak C, Li S, Bushdiecker D 2nd, Musselman IH, Pantano P, Draper RK (2011) Cytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharm 8:1351–1361
Kowalczyk B, Lagzi I, Grzybowski BA (2011) Nanoseparations: strategies for size and/or shape-selective purification of nanoparticles. Curr Opin Colloid Interface Sci 16:135–148
Lodhia J, Mandarano G, Ferris N, Eu P, Cowell S (2010) Development and use of iron oxide nanoparticles (part 1): synthesis of iron oxide nanoparticles for MRI. Biomed Imaging Interv J 6:e12
Petters C, Bulcke F, Thiel K, Bickmeyer U, Dringen R (2014) Uptake of fluorescent iron oxide nanoparticles by oligodendroglial OLN-93 cells. Neurochem Res 39:372–383
Rastedt W, Thiel K, Dringen R (2017) Uptake of fluorescent iron oxide nanoparticles in C6 glioma cells. Biomed Phys Eng Expr 3:035007
Geppert M, Hohnholt MC, Thiel K, Nurnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22:145101
Lim J, Yeap SP, Che HX, Low SC (2013) Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett 8:381–394
Drasler B, Vanhecke D, Rodriguez-Lorenzo L, Petri-Fink A, Rothen-Rutishauser B (2017) Quantifying nanoparticle cellular uptake: which method is best? Nanomedicine 12:1095–1099
Westsson E, Koper GJM (2014) How to determine the core-shell nature in bimetallic catalyst particles? Catalysts 4:375–396
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110
Stepto RFT (2009) Dispersity in polymer science. Pure Appl Chem 81:351–353
Stetefeld J, McKenna SA, Patel TR (2016) Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys Rev 8:409–427
Zhang W (2014) Nanoparticle aggregation: principles and modeling. Adv Exp Med Biol 811:19–43
Stewart PL (2017) Cryo-electron microscopy and cryo-electron tomography of nanoparticles. WIREs Nanomed Nanobiotechnol 9:e1417
Tantra R, Schulze P, Quincey P (2010) Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 8:279–285
Slater TJA, Janssen A, Camargo PHC, Burke MG, Zaluzec NJ, Haigh SJ (2016) STEM-EDX tomography of bimetallic nanoparticles: a methodological investigation. Ultramicroscopy 162:61–73
Clogston JD, Patri AK (2011) Zeta potential measurement. Methods Mol Biol 697:63–70
Rades S, Hodoroaba VD, Salge T, Wirth T, Lobera MP, Labrador RH, Natte K, Behnke T, Gross T, Unger WES (2014) High-resolution imaging with SEM/T-SEM, EDX and SAM as a combined methodical approach for morphological and elemental analyses of single engineered nanoparticles. RSC Adv 4:49577–49587
Luther EM, Petters C, Bulcke F, Kaltz A, Thiel K, Bickmeyer U, Dringen R (2013) Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater 9:8454–8465
Lopez-Lorente AI, Mizaikoff B (2016) Recent advances on the characterization of nanoparticles using infrared spectroscopy. Trends Anal Chem 84:97–106
Bertorelle F, Wilhelm C, Roger J, Gazeau F, Menager C, Cabuil V (2006) Fluorescence-modified superparamagnetic nanoparticles: intracellular uptake and use in cellular imaging. Langmuir 22:5385–5391
Herrmann R, Rennhak M, Reller A (2014) Synthesis and characterization of fluorescence-labelled silica core-shell and noble metal-decorated ceria nanoparticles. Beilstein J Nanotechnol 5:2413–2423
Geppert M, Petters C, Thiel K, Dringen R (2013) The presence of serum alters the properties of iron oxide nanoparticles and lowers their accumulation by cultured brain astrocytes. J Nanopart Res 15:1349
Petters C, Thiel K, Dringen R (2016) Lysosomal iron liberation is responsible for the vulnerability of brain microglial cells to iron oxide nanoparticles: comparison with neurons and astrocytes. Nanotoxicology 10:332–342
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 22:375101
Joshi A, Rastedt W, Faber K, Schultz AG, Bulcke F, Dringen R (2016) Uptake and toxicity of copper oxide nanoparticles in C6 glioma cells. Neurochem Res 41:3004–3019
Bulcke F, Thiel K, Dringen R (2014) Uptake and toxicity of copper oxide nanoparticles in cultured primary brain astrocytes. Nanotoxicology 8:775–785
Geppert M, Hohnholt M, Gaetjen L, Grunwald I, Baumer M, Dringen R (2009) Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J Biomed Nanotechnol 5:285–293
Waters JC (2009) Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol 185:1135–1148
Ruedas-Rama MJ, Walters JD, Orte A, Hall EA (2012) Fluorescent nanoparticles for intracellular sensing: a review. Anal Chim Acta 751:1–23
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44:4743–4768
Shevtsov MA, Nikolaev BP, Ryzhov VA, Yakovleva LY, Marchenko YY, Parr MA, Rolich VI, Mikhrina AL, Dobrodumov AV, Pitkin E, Multhoff G (2015) Ionizing radiation improves glioma-specific targeting of superparamagnetic iron oxide nanoparticles conjugated with cmHsp70.1 monoclonal antibodies (SPION-cmHsp70.1). Nanoscale 7:20652–20664
Shevtsov MA, Yakovleva LY, Nikolaev BP, Marchenko YY, Dobrodumov AV, Onokhin KV, Onokhina YS, Selkov SA, Mikhrina AL, Guzhova IV, Martynova MG, Bystrova OA, Ischenko AM, Margulis BA (2014) Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma. Neuro Oncol 16:38–49
Galli M, Guerrini A, Cauteruccio S, Thakare P, Dova D, Orsini F, Arosio P, Carrara C, Sangregorio C, Lascialfari A, Maggioni D, Licandro E (2017) Superparamagnetic iron oxide nanoparticles functionalized by peptide nucleic acids. RSC Adv 7:15500–15512
Shi D, Sun L, Mi G, Sheikh L, Bhattacharya S, Nayar S, Webster TJ (2014) Controlling ferrofluid permeability across the blood-brain barrier model. Nanotechnology 25:075101
Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074
Liang J, Horton JH (2005) Interactions of benzoic acid and phosphates with iron oxide colloids using chemical force titration. Langmuir 21:10608–10614
Ruiz A, Morais PC, de Azevedo RB, Lacava ZGM, Villanueva A, Morales MD (2014) Magnetic nanoparticles coated with dimercaptosuccinic acid: development, characterization, and application in biomedicine. J Nanopart Res 16:2589
Soler MA, Lima EC, Nunes ES, Silva FL, Oliveira AC, Azevedo RB, Morais PC (2011) Spectroscopic study of maghemite nanoparticles surface-grafted with DMSA. J Phys Chem A 115:1003–1008
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen HS (eds) Brain energy metabolism. Springer, New York, pp 45–72
Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8:543–557
Doak SH, Griffiths SM, Manshian B, Singh N, Williams PM, Brown AP, Jenkins GJS (2009) Confounding experimental considerations in nanogenotoxicology. Mutagenesis 24:285–293
Pederzoli F, Tosi G, Vandelli MA, Belletti D, Forni F, Ruozi B (2017) Protein corona and nanoparticles: how can we investigate on? Wiley Interdiscip Rev Nanomed Nanobiotechnol 9:e1467
Iversen TG, Skotland T, Sandvig K (2011) Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6:176–185
Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B (2014) Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol 5:1625–1636
Guo S, Zhang X, Zheng M, Zhang X, Min C, Wang Z, Cheon SH, Oak MH, Nah SY, Kim KM (2015) Selectivity of commonly used inhibitors of clathrin-mediated and caveolae-dependent endocytosis of G protein-coupled receptors. Biochim Biophys Acta 1848:2101–2110
Dutta D, Donaldson JG (2012) Search for inhibitors of endocytosis: intended specificity and unintended consequences. Cell Logist 2:203–208
Kirchhausen T, Macia E, Pelish HE (2008) Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol 438:77–93
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850
Kroll A, Pillukat MH, Hahn D, Schnekenburger J (2012) Interference of engineered nanoparticles with in vitro toxicity assays. Arch Toxicol 86:1123–1136
Yang WJ, Lee JH, Hong SC, Lee J, Lee J, Han DW (2013) Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 6:4689–4706
Frohlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7:5577–5591
Geppert M, Hohnholt MC, Nurnberger S, Dringen R (2012) Ferritin up-regulation and transient ROS production in cultured brain astrocytes after loading with iron oxide nanoparticles. Acta Biomater 8:3832–3839
Lamkowsky MC, Geppert M, Schmidt MM, Dringen R (2012) Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J Biomed Mater Res A 100:323–334
Zhang L, Wang X, Zou J, Liu Y, Wang J (2015) DMSA-coated iron oxide nanoparticles greatly affect the expression of genes coding cysteine-rich proteins by their DMSA coating. Chem Res Toxicol 28:1961–1974
Drexler HG, Dirks WG, MacLeod RA, Uphoff CC (2017) False and mycoplasma-contaminated leukemia-lymphoma cell lines: time for a reappraisal. Int J Cancer 140:1209–1214
Freedman LP, Gibson MC, Ethier SP, Soule HR, Neve RM, Reid YA (2015) Reproducibility: changing the policies and culture of cell line authentication. Nat Methods 12:493–497
Sunol C, Babot Z, Fonfria E, Galofre M, Garcia D, Herrera N, Iraola S, Vendrell I (2008) Studies with neuronal cells: from basic studies of mechanisms of neurotoxicity to the prediction of chemical toxicity. Toxicol In Vitro 22:1350–1355
Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD (2012) Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37:2569–2588
Gordon J, Amini S, White MK (2013) General overview of neuronal cell culture. Methods Mol Biol 1078:1–8
Lian H, Roy E, Zheng H (2016) Protocol for primary microglial culture preparation. Bio Protoc 6:e1989
Barateiro A, Fernandes A (2014) Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta 1843:1917–1929
Kaur G, Dufour JM (2012) Cell lines: valuable tools or useless artifacts. Spermatogenesis 2:1–5
Petters C, Dringen R (2014) Comparison of primary and secondary rat astrocyte cultures regarding glucose and glutathione metabolism and the accumulation of iron oxide nanoparticles. Neurochem Res 39:46–58
Humpel C (2015) Organotypic brain slice cultures: a review. Neuroscience 305:86–98
Fleige G, Nolte C, Synowitz M, Seeberger F, Kettenmann H, Zimmer C (2001) Magnetic labeling of activated microglia in experimental gliomas. Neoplasia 3:489–499
Pickard MR, Chari DM (2010) Robust uptake of magnetic nanoparticles (MNPs) by central nervous system (CNS) microglia: implications for particle uptake in mixed neural cell populations. Int J Mol Sci 11:967–981
Snijder B, Sacher R, Ramo P, Damm EM, Liberali P, Pelkmans L (2009) Population context determines cell-to-cell variability in endocytosis and virus infection. Nature 461:520–523
Wang X, Hu X, Li J, Russe AC, Kawazoe N, Yang Y, Chen G (2016) Influence of cell size on cellular uptake of gold nanoparticles. Biomater Sci 4:970–978
Kim JA, Aberg C, Salvati A, Dawson KA (2011) Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat Nanotechnol 7:62–68
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Boucrot E, Kirchhausen T (2007) Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA 104:7939–7944
Tang J, Liu Z, Ji F, Li Y, Liu J, Song J, Li J, Zhou J (2015) The role of the cell cycle in the cellular uptake of folate-modified poly(L-amino acid) micelles in a cell population. Nanoscale 7:20397–20404
Patel P, Kansara K, Senapati VA, Shanker R, Dhawan A, Kumar A (2016) Cell cycle dependent cellular uptake of zinc oxide nanoparticles in human epidermal cells. Mutagenesis 31:481–490
Roder A, Garcia-Gareta E, Theodoropoulos C, Ristovski N, Blackwood KA, Woodruff MA (2015) An assessment of cell culture plate surface chemistry for in vitro studies of tissue engineering scaffolds. J Funct Biomater 6:1054–1063
Curtis AS, Forrester JV, McInnes C, Lawrie F (1983) Adhesion of cells to polystyrene surfaces. J Cell Biol 97:1500–1506
Aday S, Hasirci N, Gurhan I (2011) A cost-effective and simple culture method for primary hepatocytes. Anim Cells Syst 15:19–27
Salzig D, Leber J, Merkewitz K, Lange MC, Koster N, Czermak P (2016) Attachment, growth, and detachment of human mesenchymal stem cells in a chemically defined medium. Stem Cells Int 2016:5246584
Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B (2011) Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta 1810:361–373
Dunning MD, Lakatos A, Loizou L, Kettunen M, ffrench-Constant C, Brindle KM, Franklin RJ (2004) Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci 24:9799–9810
Villanueva A, Canete M, Roca AG, Calero M, Veintemillas-Verdaguer S, Serna CJ, Morales Mdel P, Miranda R (2009) The influence of surface functionalization on the enhanced internalization of magnetic nanoparticles in cancer cells. Nanotechnology 20:115103
Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K (2018) Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep 8:2082
Sun Z, Yathindranath V, Worden M, Thliveris JA, Chu S, Parkinson FE, Hegmann T, Miller DW (2013) Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int J Nanomed 8:961–970
Chen J, Zhu JM, Cho HH, Cui KM, Li FH, Zhou XB, Rogers JT, Wong STC, Huang XD (2008) Differential cytotoxicity of metal oxide nanoparticles. J Exp Nanosci 3:321–328
Warheit DB (2008) How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicol Sci 101:183–185
Hong SC, Lee JH, Lee J, Kim HY, Park JY, Cho J, Lee J, Han DW (2011) Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int J Nanomed 6:3219–3231
Rivet CJ, Yuan Y, Borca-Tasciuc DA, Gilbert RJ (2012) Altering iron oxide nanoparticle surface properties induce cortical neuron cytotoxicity. Chem Res Toxicol 25:153–161
Kroll A, Pillukat MH, Hahn D, Schnekenburger J (2009) Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur J Pharm Biopharm 72:370–377
Hohnholt MC, Blumrich EM, Dringen R (2015) Multiassay analysis of the toxic potential of hydrogen peroxide on cultured neurons. J Neurosci Res 93:1127–1137
Stone V, Johnston H, Schins RPF (2009) Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol 39:613–626
Galdiero S, Falanga A, Morelli G, Galdiero M (2015) gH625: a milestone in understanding the many roles of membranotropic peptides. Biochim Biophys Acta 1848:16–25
Almeida A, Delgado-Esteban M, Bolanos JP, Medina JM (2002) Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem 81:207–217
Hoskins C, Wang LJ, Cheng WP, Cuschieri A (2012) Dilemmas in the reliable estimation of the in-vitro cell viability in magnetic nanoparticle engineering: which tests and what protocols? Nanoscale Res Lett 7:1–12
Wilhelm C, Gazeau F, Roger J, Pons JN, Bacri JC (2002) Interaction of anionic superparamagnetic nanoparticles with cells: kinetic analyses of membrane adsorption and subsequent internalization. Langmuir 18:8148–8155
Wilhelm C, Billotey C, Roger J, Pons JN, Bacri JC, Gazeau F (2003) Intracellular uptake of anionic superparamagnetic nanoparticles as a function of their surface coating. Biomaterials 24:1001–1011
Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Aberg C (2013) Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 135:1438–1444
Pelaz B, Charron G, Pfeiffer C, Zhao Y, de la Fuente JM, Liang XJ, Parak WJ, Del Pino P (2013) Interfacing engineered nanoparticles with biological systems: anticipating adverse nano-bio interactions. Small 9:1573–1584
Petters C, Dringen R (2015) Accumulation of iron oxide nanoparticles by cultured primary neurons. Neurochem Int 81:1–9
Hohnholt MC, Geppert M, Dringen R (2011) Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater 7:3946–3954
Calero M, Chiappi M, Lazaro-Carrillo A, Rodriguez MJ, Chichon FJ, Crosbie-Staunton K, Prina-Mello A, Volkov Y, Villanueva A, Carrascosa JL (2015) Characterization of interaction of magnetic nanoparticles with breast cancer cells. J Nanobiotechnol 13:16
dos Santos T, Varela J, Lynch I, Salvati A, Dawson KA (2011) Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 6:e24438
Pilakka-Kanthikeel S, Atluri VS, Sagar V, Saxena SK, Nair M (2013) Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in-vitro study. PLoS ONE 8:e62241
Ahrens ET, Feili-Hariri M, Xu H, Genove G, Morel PA (2003) Receptor-mediated endocytosis of iron-oxide particles provides efficient labeling of dendritic cells for in vivo MR imaging. Magn Reson Med 49:1006–1013
Lachowicz D, Szpak A, Malek-Zietek KE, Kepczynski M, Muller RN, Laurent S, Nowakowska M, Zapotoczny S (2017) Biocompatible and fluorescent superparamagnetic iron oxide nanoparticles with superior magnetic properties coated with charged polysaccharide derivatives. Colloids Surf B 150:402–407
Rejman J, Nazarenus M, de Aberasturi DJ, Said AH, Feliu N, Parak WJ (2016) Some thoughts about the intracellular location of nanoparticles and the resulting consequences. J Colloid Interface Sci 482:260–266
Zhang YQ, Dringen R, Petters C, Rastedt W, Koser J, Filser J, Stolte S (2016) Toxicity of dimercaptosuccinate-coated and un-functionalized magnetic iron oxide nanoparticles towards aquatic organisms. Environ Sci Nano 3:754–767
Mazuel F, Espinosa A, Luciani N, Reffay M, Le Borgne R, Motte L, Desboeufs K, Michel A, Pellegrino T, Lalatonne Y, Wilhelm C (2016) Massive intracellular biodegradation of iron oxide nanoparticles evidenced magnetically at single-endosome and tissue levels. ACS Nano 10:7627–7638
Skotland T, Sontum PC, Oulie I (2002) In vitro stability analyses as a model for metabolism of ferromagnetic particles (Clariscan), a contrast agent for magnetic resonance imaging. J Pharm Biomed Anal 28:323–329
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc 2:223–228
Acknowledgements
The authors thank Dr. Karsten Thiel (Fraunhofer IFAM, Bremen) for kindly providing the TEM picture of the DMSA-IONPs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Willmann, W., Dringen, R. How to Study the Uptake and Toxicity of Nanoparticles in Cultured Brain Cells: The Dos and Don’t Forgets. Neurochem Res 44, 1330–1345 (2019). https://doi.org/10.1007/s11064-018-2598-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2598-4