Abstract
Introduction
Our recent work reported that GOLPH3 promotes glioma progression via inhibiting endocytosis and degradation of EGFR. The current study aimed to explore the potential regulating mechanism of GOLPH3 on JAK2–STAT3 signaling, a downstream effector of EGFR, in glioma progression.
Methods
The expression of JAK2, STAT3 and GOLPH3 in glioma tissues was detected by western blotting, tissue microarray and immunohistochemistry. The U251 and U87 cells with GOLPH3 down-regulation or over-expression were generated by lentivirus system. The effects of GOLPH3 on the activity of JAK2 and STAT3 were detected by western blotting and reverse transcription polymerase chain reaction. Co-immunoprecipitation was used to detect the association of GOLPH3 with JAK2 and STAT3. Cell proliferation was detected by CCK8 and EdU assay.
Results
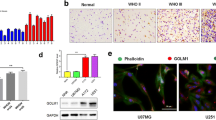
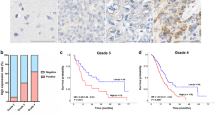
The level of JAK2, STAT3 and GOLPH3 were significantly up-regulated and exhibited pairwise correlation in human glioma tissues. The level of p-JAK2 and p-STAT3, as well as the mRNA and protein levels of cyclin D1 and c-myc, two target genes of STAT3, decreased after GOLPH3 down-regulation, while they increased after GOLPH3 over-expression both in U251 and U87 cells. Interestingly, GOLPH3, JAK2 and STAT3 existed in the same protein complex and GOLPH3 affected the interaction of JAK2 and STAT3. Importantly, down-regulation of STAT3 partially abolished cell proliferation induced by GOLPH3 over-expression.
Conclusions
GOLPH3 may act as a scaffold protein to regulate JAK2–STAT3 interaction and then its activation, which therefore mediates the effect of GOLPH3 on cell proliferation.





Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507
Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG (2009) PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J Cell Biol 187(7):967–975
Sechi S, Frappaolo A, Belloni G, Colotti G, Giansanti MG (2015) The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3. Oncotarget 6(6):3493–3506
Snyder CM, Mardones GA, Ladinsky MS, Howell KE (2006) GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi. Mol Biol Cell 17(1):511–524
Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE (2000) GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic 1(12):963–975
Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB (1999) Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem 274(48):34294–34300
Farber-Katz SE, Dippold HC, Buschman MD et al (2014) DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 156(3):413–427
Walch-Solimena C, Novick P (1999) The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol 1(8):523–525
Ciccia A, Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40(2):179–204
McKay HF, Burgess DR (2011) ‘Life is a highway’: membrane trafficking during cytokinesis. Traffic 12(3):247–251
Scott KL, Kabbarah O, Liang M-C et al (2009) GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 459(7250):1085–1090
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126(5):855–867
Scott KL, Chin L (2010) Signaling from the Golgi: mechanisms and models for Golgi phosphoprotein 3-mediated oncogenesis. Clin Cancer Res 16(8):2229–2234
Ali MF, Chachadi VB, Petrosyan A, Cheng PW (2012) Golgi phosphoprotein 3 determines cell binding properties under dynamic flow by controlling Golgi localization of core 2 N-acetylglucosaminyltransferase 1. J Biol Chem 287(47):39564–39577
Tu L, Chen L, Banfield DK (2012) A conserved N-terminal arginine-motif in GOLPH3-family proteins mediates binding to coatomer. Traffic 13(11):1496–1507
Tu L, Tai WC, Chen L, Banfield DK (2008) Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science 321(5887):404–407
Rosnoblet C, Peanne R, Legrand D, Foulquier F (2013) Glycosylation disorders of membrane trafficking. Glycoconj J 30(1):23–31
Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science 291(5512):2364–2369
Zhou X, Xue P, Yang M et al (2014) Protein kinase D2 promotes the proliferation of glioma cells by regulating Golgi phosphoprotein 3. Cancer Lett 355(1):121–129
Chiu R, Novikov L, Mukherjee S, Shields D (2002) A caspase cleavage fragment of p115 induces fragmentation of the Golgi apparatus and apoptosis. J Cell Biol 159(4):637–648
Zhang X, Ding Z, Mo J et al (2015) GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol Carcinog 54(11):1252–1263
Zhou X, Zhan W, Bian W et al (2013) GOLPH3 regulates the migration and invasion of glioma cells though RhoA. Biochem Biophys Res Commun 433(3):338–344
Zhou X, Xie S, Wu S et al (2017) Golgi phosphoprotein 3 promotes glioma progression via inhibiting Rab5-mediated endocytosis and degradation of epidermal growth factor receptor. Neuro-oncology 19(12):1628–1639
Felsberg J, Hentschel B, Kaulich K et al (2017) Epidermal Growth Factor Receptor Variant III (EGFRvIII) positivity in EGFR-amplified glioblastomas: prognostic role and comparison between primary and recurrent tumors. Clin Cancer Res 23(22):6846–6855
Quesnelle KM, Boehm AL, Grandis JR (2007) STAT-mediated EGFR signaling in cancer. J Cell Biochem 102(2):311–319
Fan QW, Cheng CK, Gustafson WC et al (2013) EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 24(4):438–449
Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264(5164):1415–1421
Heppler LN, Frank DA (2017) Targeting oncogenic transcription factors: therapeutic implications of endogenous STAT inhibitors. Trends Cancer 3(12):816–827
Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19(21):2474–2488
de la Iglesia N, Konopka G, Lim KL et al (2008) Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci 28(23):5870–5878
Bromberg JF, Darnell JE Jr. (1999) Potential roles of Stat1 and Stat3 in cellular transformation. Cold Spring Harb Symp Quant Biol 64:425–428
Besser D, Bromberg JF, Darnell JE Jr, Hanafusa H (1999) A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol Cell Biol 19(2):1401–1409
Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R (2000) Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19(48):5419–5427
Yang J, Stark GR (2008) Roles of unphosphorylated STATs in signaling. Cell Res 18(4):443–451
Yan SJ, Lim SJ, Shi S, Dutta P, Li WX (2011) Unphosphorylated STAT and heterochromatin protect genome stability. FASEB J 25(1):232–241
Colomiere M, Ward AC, Riley C et al (2008) Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial–mesenchymal transition in ovarian carcinomas. Br J Cancer 100(1):134–144
Zeng Z, Lin H, Zhao X et al (2012) Overexpression of GOLPH3 promotes proliferation and tumorigenicity in breast cancer via suppression of the FOXO1 transcription factor. Clin Cancer Res 18(15):4059–4069
Dai T, Zhang D, Cai M et al (2015) Golgi phosphoprotein 3 (GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by activating the NF-κB pathway. J Pathol 235(3):490–501
Jin H, Pi J, Zhao Y et al (2017) EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale 9(42):16365–16374
Blakely CM, Watkins TBK, Wu W et al (2017) Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 49(12):1693–1704
Runge D, Runge DM, Drenning SD, Bowen WC Jr, Grandis JR, Michalopoulos GK (1998) Growth and differentiation of rat hepatocytes: changes in transcription factors HNF-3, HNF-4, STAT-3, and STAT-5. Biochem Biophys Res Commun 250(3):762–768
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant Nos: 81672489; 81472345); Six Major Talent Summit of Jiangsu Province (Grant No: 2014-WSW-039). We thank professor Chunmei Zhu in School of International Education of Xuzhou Medical University for English writing assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, S., Fu, J., Dong, Y. et al. GOLPH3 promotes glioma progression via facilitating JAK2–STAT3 pathway activation. J Neurooncol 139, 269–279 (2018). https://doi.org/10.1007/s11060-018-2884-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2884-7