Abstract
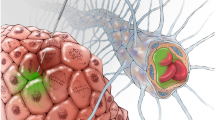
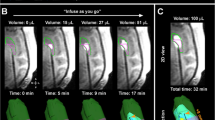
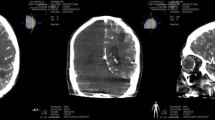
Cetuximab conjugated iron-oxide nanoparticles (cetuximab-IONPs) have shown both in-vitro and in-vivo anti-tumor efficacy against gliomas. The purpose of this pilot study was to evaluate the safety and potential efficacy of cetuximab-IONPs for treatment of spontaneously occurring intracranial gliomas in canines after convection-enhanced delivery (CED). The use of CED allowed for direct infusion of the cetuximab-IONPs both intratumorally and peritumorally avoiding the blood brain barrier (BBB) and limiting systemic effects. A total of eight dogs participated in the study and only two developed mild post-operative complications, which resolved with medical therapy. All canines underwent a single CED treatment of the cetuximab-IONPs over 3 days and did not receive any further adjuvant treatments. Volumetric analysis showed a median reduction in tumor size of 54.9% by MRI at 1-month (4–6 weeks) follow-up. Five dogs were euthanized due to recurrence of neurological signs other than seizures, two due to recurrent seizures, and one dog died in his sleep. Median survival time after surgery was 248 days (mean 367 days).




Similar content being viewed by others
Notes
GE 3.0T Signa HDx; GE Healthcare, Milwaukee, WI.
3.0T Siemens Skyra.
Siemens Symphony, TIM technology.
Butorphanol tartrate; Torbugesic®, Fort Dodge, Fort Dodge, IA.
Diazepam, Valium®; Hospira, Lakeforest, IL.
Atropine sulfate; Med Pharmex, Pomona, CA.
Glycopyrrolate; Baxter Healthcare Corp., Deerfield, IL.
PropoFlo; Abbott Laboratories, North Chicago, IL.
Isoflurane, MDI, Boise, ID.
Gadopentetate dimeglumine, Magnevist®; Bayer HealthCare Pharmaceuticals, Wayne, NJ.
Medtronic, Inc., Minneapolis, MN.
See footnote 11.
See footnote 11.
OsiriX 3.6, Pixmeo, Bernex, Switzerland.
See footnote 14
See footnote 14
Rabbit anti-olig2; GeneTex, Irvine, CA.
Mouse anti-GFAP, Biogenex, San Ramon, CA.
Mouse anti-EGFR, Lifespan Biosciences.
Biotinylated secondary antibodies, Vector Laboratories, Burlingame, CA.
A streptavidin-HRP conjugated label, Biocare Medical, LLC, Concord, CA.
DAB, DAKO, Carpinteria, CA.
Isotype rabbit or mouse control serum, Biocare Medical, LLC, Concord, CA.
References
Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C (2006) Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med 20:669–675
Song RB, Vite CH, Bradley CW, Cross JR (2013) Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med 27:1143–1152. https://doi.org/10.1111/jvim.12136
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(Suppl 5): v1–v49. https://doi.org/10.1093/neuonc/nos218
Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899. https://doi.org/10.1158/0008-5472.CAN-04-1337
Hu H, Barker A, Harcourt-Brown T, Jeffery N (2015) Systematic review of brain tumor treatment in dogs. J Vet Intern Med 29:1456–1463. https://doi.org/10.1111/jvim.13617
Rossmeisl JH, Duncan RB, Huckle WR, Troy GC (2007) Expression of vascular endothelial growth factor in tumors and plasma from dogs with primary intracranial neoplasms. Am J Vet Res 68:1239–1245. https://doi.org/10.2460/ajvr.68.11.1239
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Larson RF, Yamashita Y, Krauze MT, Forsayeth J, Noble CO, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2010) Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol 12:928–940. https://doi.org/10.1093/neuonc/noq046
Stoica G, Kim HT, Hall DG, Coates JR (2004) Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol 41:10–19. https://doi.org/10.1354/vp.41-1-10
Chakravarti A, Dicker A, Mehta M (2004) The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: a review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys 58:927–931. https://doi.org/10.1016/j.ijrobp.2003.09.092
Sathornsumetee S, Rich JN (2008) Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci 1142:108–132. https://doi.org/10.1196/annals.1444.009
Zhang X, Zhang W, Cao WD, Cheng G, Zhang YQ (2012) Glioblastoma multiforme: Molecular characterization and current treatment strategy (review). Exp Ther Med 3:9–14. https://doi.org/10.3892/etm.2011.367
Boudreau CE, York D, Higgins RJ, LeCouteur RA, Dickinson PJ (2017) Molecular signalling pathways in canine gliomas. Vet Comp Oncol 15:133–150. https://doi.org/10.1111/vco.12147
Dickinson PJ (2014) Advances in diagnostic and treatment modalities for intracranial tumors. J Vet Intern Med 28:1165–1185. https://doi.org/10.1111/jvim.12370
Higgins RJ, Dickinson PJ, LeCouteur RA, Bollen AW, Wang H, Wang H, Corely LJ, Moore LM, Zang W, Fuller GN (2010) Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neuro-oncol 98:49–55. https://doi.org/10.1007/s11060-009-0072-5
Debinski W, Dickinson P, Rossmeisl JH, Robertson J, Gibo DM (2013) New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS ONE 8:e77719. https://doi.org/10.1371/journal.pone.0077719
York D, Sproul CD, Chikere N, Dickinson PJ, Angelastro JM (2017) Expression and targeting of transcription factor ATF5 in dog gliomas. Vet Comp Oncol. https://doi.org/10.1111/vco.12317
Dickinson PJ, Roberts BN, Higgins RJ, Leutenegger CM, Bollen AW, Kass PH, LeCouteur RA (2006) Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet Comp Oncol 4:132–140. https://doi.org/10.1111/j.1476-5829.2006.00101.x
Neyns B, Sadones J, Joosens E, Bouttens F, Verbeke L, Baurain JF, D’Hondt L, Strauven T, Chaskis C, In’t Veld P, Michotte A, De Greve J (2009) Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol 20: 1596–1603. https://doi.org/10.1093/annonc/mdp032
Dunn IF, Heese O, Black PM (2000) Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neuro-oncol 50:121–137
Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B (1987) Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA 84:6899–6903
Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O’Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB (2001) PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst 93:1246–1256
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455: 1061–1068. https://doi.org/10.1038/nature07385
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Jutten B, Dubois L, Li Y, Aerts H, Wouters BG, Lambin P, Theys J, Lammering G (2009) Binding of cetuximab to the EGFRvIII deletion mutant and its biological consequences in malignant glioma cells. Radiother Oncol 92:393–398. https://doi.org/10.1016/j.radonc.2009.06.021
Combs SE, Heeger S, Haselmann R, Edler L, Debus J, Schulz-Ertner D (2006) Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)—phase I/II trial: study protocol. BMC Cancer 6:133. https://doi.org/10.1186/1471-2407-6-133
Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, Wrba F, Horvat R, Thalhammer JG, Willmann M, Jensen-Jarolim E (2012) Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol 50:200–209. https://doi.org/10.1016/j.molimm.2012.01.002
Baumann M, Krause M (2004) Targeting the epidermal growth factor receptor in radiotherapy: radiobiological mechanisms, preclinical and clinical results. Radiother Oncol 72:257–266. https://doi.org/10.1016/j.radonc.2004.07.007
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, Broholm H, Stockhausen MT, Poulsen HS (2010) Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol 12:508–516. https://doi.org/10.1093/neuonc/nop063
Belda-Iniesta C, Carpeno Jde C, Saenz EC, Gutierrez M, Perona R, Baron MG (2006) Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther 5:912–914
Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, Wu X, Mao H (2010) EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 70:6303–6312. https://doi.org/10.1158/0008-5472.CAN-10-1022
Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, Tseng IC, Wang JJ, Yen TC, Chen PY, Wei KC (2010) Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA 107:15205–15210. https://doi.org/10.1073/pnas.1003388107
Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG (2015) Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 6:8788–8806. https://doi.org/10.18632/oncotarget.3554
Barua NU, Gill SS, Love S (2013) Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathology. https://doi.org/10.1111/bpa.12082
Yun J, Rothrock RJ, Canoll P, Bruce JN (2013) Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv 2013:107573. https://doi.org/10.1155/2013/107573
Saito R, Tominaga T (2012) Convection-enhanced delivery: from mechanisms to clinical drug delivery for diseases of the central nervous system. Neurol Med Chir 52:531–538
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Roberts B, Larson RF, Yamashita Y, Krauze M, Noble CO, Drummond D, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2008) Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg 108:989–998. https://doi.org/10.3171/JNS/2008/108/5/0989
Platt S, Nduom E, Kent M, Freeman C, Machaidze R, Kaluzova M, Wang L, Mao H, Hadjipanayis CG (2012) Canine model of convection-enhanced delivery of cetuximab-conjugated iron-oxide nanoparticles monitored with magnetic resonance imaging. Clin Neurosurg 59:107–113. https://doi.org/10.1227/NEU.0b013e31826989ef
Young BD, Levine JM, Porter BF, Chen-Allen AV, Rossmeisl JH, Platt SR, Kent M, Fosgate GT, Schatzberg SJ (2011) Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound 52:132–141. https://doi.org/10.1111/j.1740-8261.2010.01758.x
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Martens T, Laabs Y, Gunther HS, Kemming D, Zhu Z, Witte L, Hagel C, Westphal M, Lamszus K (2008) Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res 14:5447–5458. https://doi.org/10.1158/1078-0432.CCR-08-0147
Diaz Miqueli A, Rolff J, Lemm M, Fichtner I, Perez R, Montero E (2009) Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer 100:950–958. https://doi.org/10.1038/sj.bjc.6604943
Eller JL, Longo SL, Hicklin DJ, Canute GW (2002) Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 51:1005–1013 (discussion 1013-1004)
Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW (2005) Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery 56:155–162 (discussion 162)
Chakraborty S, Filippi CG, Wong T, Ray A, Fralin S, Tsiouris AJ, Praminick B, Demopoulos A, McCrea HJ, Bodhinayake I, Ortiz R, Langer DJ, Boockvar JA (2016) Superselective intraarterial cerebral infusion of cetuximab after osmotic blood/brain barrier disruption for recurrent malignant glioma: phase I study. J Neuro-oncol 128:405–415. https://doi.org/10.1007/s11060-016-2099-8
Garrett CR, Eng C (2011) Cetuximab in the treatment of patients with colorectal cancer. Expert Opin Biol Ther 11:937–949. https://doi.org/10.1517/14712598.2011.582464
Hebbar M, Wacrenier A, Desauw C, Romano O, Cattan S, Triboulet JP, Pruvot FR (2006) Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer Drugs 17:855–857. https://doi.org/10.1097/01.cad.0000217425.44584.9f
Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB (2005) Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol 23:1803–1810. https://doi.org/10.1200/JCO.2005.08.037
Sandhiya S, Dkhar SA, Surendiran A (2009) Emerging trends of nanomedicine–an overview. Fundam Clin Pharmacol 23:263–269. https://doi.org/10.1111/j.1472-8206.2009.00692.x
Mahmoudi K, Hadjipanayis CG (2014) The application of magnetic nanoparticles for the treatment of brain tumors. Front Chem 2:109. https://doi.org/10.3389/fchem.2014.00109
Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG (2012) Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol 5:173–186. https://doi.org/10.1586/ecp.12.1
Bouras A, Kaluzova M, Hadjipanayis CG (2015) Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neuro-oncol 124:13–22. https://doi.org/10.1007/s11060-015-1807-0
Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, Brady ML, Reardon DA, Friedman AH, Friedman HS, Rodriguez-Ponce MI, Chang SM, Mittermeyer S, Croteau D, Puri RK, Investigators PT (2010) Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–309. https://doi.org/10.3171/2009.11.JNS091052
Lipsitz D, Higgins RJ, Kortz GD, Dickinson PJ, Bollen AW, Naydan DK, LeCouteur RA (2003) Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol 40:659–669. https://doi.org/10.1354/vp.40-6-659
Dolera M, Malfassi L, Bianchi C, Carrara N, Finesso S, Marcarini S, Mazza G, Pavesi S, Sala M, Urso G (2017) Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet Comp Oncol. https://doi.org/10.1111/vco.12316
Acknowledgements
The authors thank Ken Johnson with the Boo Radley Foundation, Wilder Grummon with Medtronics, the UGA Bioimaging Research Center, Lisa Reno, Tim Jarrett, Kim Mason, referral neurologists including Gillian Irving and Jason King.
Funding
Funding provided in part by American Kennel Club Canine Health Foundation, the NIH (NS053454; P50CA128301-01A10003), the Georgia Cancer Coalition, Distinguished Cancer Clinicians and Scientists Program, and the Dana Foundation, the Boo Radley Foundation, and UGA clinical research grants.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Freeman, A.C., Platt, S.R., Holmes, S. et al. Convection-enhanced delivery of cetuximab conjugated iron-oxide nanoparticles for treatment of spontaneous canine intracranial gliomas. J Neurooncol 137, 653–663 (2018). https://doi.org/10.1007/s11060-018-2764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2764-1