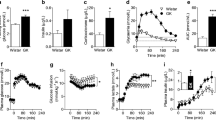
Studies in ICR mice using proton magnetic resonance spectroscopy (1H MRS) addressed the metabolomic reaction of the hippocampus to deficiency of available energy induced by administration of 2-deoxy-Dglucose (2DG) and activation of the mechanisms of inflammation due to injection of bacterial lipopolysaccharide (LPS). Administration of 2DG increased the proportion of excitatory metabolites (glutamate and glutamine) and decreased the proportions of the inhibitory transmitter gamma-aminobutyric acid (GABA) and metabolites involved in neurogenesis (N-acetylaspartate (NAA) and choline compounds). Administration of LPS, conversely, increased the levels of GABA, NAA, and choline compounds. The differently directed effects of energy deficiency and proinflammatory stimuli explain the contradictory nature of 1H MRS results in patients with neurodegenerative pathologies, which in most cases are combined with mitochondrial dysfunction and inflammatory processes. The predominance of excitatory metabolites after administration of 2DG and inhibitory metabolites after administration of LPS are in good agreement with behavioral reactions seen in acute energy deficiencies, for example in hypoxia and illness behavior induced by proinflammatory factors.
Similar content being viewed by others
References
A. E. Akulov, D. V. Petrovskii, and M. N. Moshkin, “The energy cost of social behavior in mice on activation of nonspecific immunity,” Zh. Obshch. Biol., 70, No. 4, 275–284 (2009).
V. A. Negovskii, Current Problems in Intensive Care [in Russian], Meditsina, Moscow (1971).
S. Rose, The Making of Memory: From Molecules to Mind [Russian translation], Mir, Moscow (1995).
T. Akema, D. He, and H. Sugiyama, “Lipopolysaccharide increases gamma-aminobutyric acid synthesis in medial preoptic neurones in association with inhibition of steroid-inducing luteinising hormone surge in female rats,” J. Neuroendocrinol., 17, No. 10, 672–678 (2005).
C. D. Albright, A. Y. Tsai, C. B. Friedrich, et al., “Choline availability alters embryonic development of the hippocampus and septum in the rat,” Brain Res. Dev. Brain Res., 113, 13–20 (1999).
D. E. Befroy, K. F. Petersen, S. Dufour, et al., “Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals,” Proc. Natl. Acad. Sci. USA, 105, No. 43, 16701–16706 (2008).
H. Benveniste, J. Drejer, A. Schousboe, and N. H. Diemer, “Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis,” J. Neurochem., 43, No. 5, 1369–1374 (1984).
J. Berlicki, “Inhibitors of glutamine synthetase and their potential applications in medicine,” Mini Rev. Med. Chem., 8, No. 9, 869–878 (2008).
D. L. Birken and W. H. Oldendorf, “N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain,” Neurosci. Biobehav. Rev., 13, No. 1, 23–31 (1989).
J. B. Blass, R. K. Sheu, and G. E. Gibson, “Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise,” Ann. N.Y. Acad. Sci., 903, 204–221 (2000).
J. Brown, “Effect of 2-deoxyglucose on carbohydrate metabolism: review of the literature and studies in the rat,” Metabolism, 11, 1098–1112 (1962).
S. Busquets, D. Sanchís, B. Alvarez, et al., “In the rat, tumor necrosis factor alpha administration results in an increase in both UCP2 and UCP3 mRNAs in skeletal muscle: a possible mechanism for cytokine-induced thermogenesis?” FEBS Lett., 440, No. 3, 348–350 (1998).
M. C. Cho, D. H. Lee, E. J. Kim, et al., “Novel PPARγ partial agonists with weak activity and no cytotoxicity identify by a simple PPARγ ligand screening system,” Mol. Cell Biochem., 358, No. 1–2, 75–83 (2011).
H. Cortez-Pinto, S. Q. Yang, H. Z. Lin, et al., “Bacterial lipopolysaccharide induces uncoupling protein-2 expression in hepatocytes by a tumor necrosis factor-alpha-dependent mechanism,” Biochem. Biophys. Res. Commun., 251, No. 1, 313–319 (1998).
R. Dantzer, “Cytokine-induced sickness behavior: Where do we stand?” Brain Behav. Immun., 15, 7–24 (2001).
D. R. Deshmukh and M. S. Patel, “Age-dependent changes to glutamate oxidation by non-synaptic and synaptic mitochondria from rat brain,” Mech. Ageing Dev., 13, No. 1, 75–81 (1980).
D. R. Donohue and S. J. Bultman, “Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression,” J. Cell Physiol., 227, No. 9, 3169–3177 (2012).
M. J. During, P. Leone, K. E. Davis, et al., “Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels,” J. Clin. Invest., 95, No. 5, 2403–2408 (1995).
S. Eklind, C. Mallard, A. L. Leverin, et al., “Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury,” Eur. J. Neurosci., 13, 1101–1106 (2001).
U. E. Emir and P. J. Tuite, “Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS,” PLoS One, 7, No. 1, 30918 (2012).
M. J. Glenn, E. M. Gibson, E. D. Kirby, et al., “Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats,” Eur. J. Neurosci., 25, No. 8, 2473–2482 (2007).
I. Hancu, E. A. Zimmerman, N. Sailasuta, and R. E. Hurd, “1H MRspectroscopy using TE averaged PRESS: A more sensitive technique to detect neurodegeneration associated with Alzheimer’s disease,” Magn. Reson. Med., 53, 777–782 (2005).
A. S. Harms, J. K. Lee, T. A. Nguyen, et al., “Regulation of microglia effector functions by tumor necrosis factor signaling,” Glia, 60, No. 2, 189–202 (2012).
F. Jessen, O. Gur, W. Block, et al., “A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI,” Neurology, 72, 1735–1740 (2009).
S. C. Lee, W. Liu, D. W. Dickson, et al., “Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta,” J. Immunol., 150, No. 7, 2659–2667 (1993).
D. L. Martin, “Regulatory properties of brain glutamate decarboxylase,” Cell Mol. Neurobiol., 7, No. 3, 237–253 (1987).
M. P. Mattson and D. Liu, “Mitochondrial potassium channels and uncoupling proteins in synaptic plasticity and neuronal cell death,” Biochem. Biophys. Res. Commun., 304, No. 3, 539–549 (2003).
B. Meldrum, “Amino acid neurotransmitters and new approaches to anticonvulsant drug action,” Epilepsia, 2, 140–149 (1984).
R. L. Melnick, L. G. Monti, and S. M. Motzkin, “Uncoupling of mitochondrial oxidative phosphorylation by thallium,” Biochem. Biophys. Res. Commun., 69, No. 1, 68–73 (1976).
J. Pfeuffer, I. Tkac, S. W. Provencher, and R. Gruetter, “Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain,” J. Magn. Reson., 141, No. 1, 104–120 (1999).
S. W. Provencher, “Estimation of metabolite concentrations from localized in vivo proton NMR spectra,” Magn. Reson. Med., 30, No. 6, 672–679 (1993).
C. Rink, S. Gnyawali, L. Peterson, and S. Khanna, “Oxygen-inducible glutamate oxcaloacetate transaminase as protective switch transforming neurotoxic glutamate to metabolic fuel during acute ischemic stroke,” Antioxid. Redox Signal., 14, No. 10, 1777–1785 (2011).
M. Sawada, N. Kondo, A. Suzumura, and T. Marunouchi, “Production of tumor necrosis factor-alpha by microglia and astrocytes in culture,” Brain Res., 491, No. 2, 394–397 (1989).
A. Shiino, T. Watanabe, Y. Shirakashi, et al., “The profile of hippocampal metabolites differs between Alzheimer’s disease and subcortical ischemic vascular dementia, as measured by proton magnetic resonance spectroscopy,” J. Cereb. Blood Flow Metab., 32, 805–815 (2012).
O. Vergun, Y. Y. Han, and I. J. Reynolds, “Glucocorticoid deprivation produces a prolonged increase in sensitivity to glutamate in cultured rat cortical neurons,” Exp. Neurol., 183, No. 2, 682–694 (2003).
A. D. B. Waldman, G. S. Rai, J. R. McConnell, et al., “Clinical brain proton magnetic resonance spectroscopy for management of Alzheimer’s and subcortical ischemic vascular dementia in older people,” Arch. Gerontol. Geriatr., 35, 137–142 (2002).
X. Wang, D. W. Carmichael, E. B. Cady, et al., “Greater hypoxiainduced cell death in prenatal brain after bacterial-endotoxin pretreatment is not because of enhanced cerebral energy depletion: a chicken embryo model of the intrapartum response to hypoxia and infection,” J. Cereb. Blood Flow Metab., 28, 948–960 (2008).
X. J. Yang, L. M. Kow, D. W. Pfaff, and C. V. Mobbs, “Metabolic pathways that mediate inhibition of hypothalamic neurons by glucose,” Diabetes, 53, No. 1, 67–73 (2004).
J. Yao, S. Chen, Z. Mao, et al., “2-Deoxy-D-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer’s disease,” PLoS One, 6, No. 7, 21788 (2011).
J. Yao, R. W. Irwin, L. Zhao, et al., “Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease,” Proc. Natl. Acad. Sci. USA, 106, 14670–14675 (2009).
W. Ying, “NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences,” Antioxid. Redox Signal., 10, No. 2, 179–206 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 98, No. 10, pp. 1264–1272, October, 2012.
Rights and permissions
About this article
Cite this article
Moshkin, M.P., Akulov, A.E., Petrovskii, D.V. et al. Magnetic Resonance Spectroscopy of Metabolic Changes in the Brains of Mice Given 2-Deoxy-D-Glucose and Lipopolysaccharide. Neurosci Behav Physi 44, 593–598 (2014). https://doi.org/10.1007/s11055-014-9956-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-9956-8