Abstract
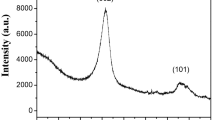
In this study, carbon nanotubes (CNTs) were successfully fabricated using a novel spark plasma–assisted pyrolysis (SPAP) method at 700–900 °C. The existence of CNTs was confirmed in all samples, the diameter of the CNTs is approximately 40–50 nm. The CNTs fabricated at 750 °C presented a helical shape, which is different from the CNTs fabricated at other temperatures which presented a bending shape. The defects of CNTs, such as bending and abnormal layer spacing, are explained through the TEM observations. The sharp diffraction peak from the XRD result and the ID/IG ratio (a minimum of 0.772) from the Raman result revealed that the CNTs prepared at higher temperature possess better crystallinity. The photoluminescence results show that the CNTs produced exhibited strong emission peaks, and the intensity of photoluminescence could be enhanced by increasing the preparation temperature. The highlighted technique is capable of achieving the preparation of CNTs quickly, safely, and inexpensively. This technique has provided a new possibility for other materials of preparation such as boron nitride nanotubes.









Similar content being viewed by others
References
Abdellatif MH, El-Komy GM, Azab AA (2018) Crystal field distortion of La3+ ion-doped Mn-Cr ferrite. J Magn Magn Mater 447:15–20
Allaedini G, Aminayi P, Tasirin SM (2016) Methane decomposition for carbon nanotube production: optimization of the reaction parameters using response surface methodology. Chem Eng Res Des 112:163–174
Allaedini G, Masrinda S, Aminayi TP (2015) Synthesis of CNTs via chemical vapor deposition of carbon dioxide as a carbon source in the presence of Ni-MgO. J Alloys Comp 647:809–814
Bacon R (1960) Growth, structure, and properties of graphite whiskers. J Appl Phys 31:283–290
Chong CT, Tan WH, Lee SL (2017) Morphology and growth of carbon nanotubes catalytically synthesized by premixed hydrocarbon-rich flames. Mater Chem Phys 197:246–255
Dileo RA, Landi BJ, Raffaelle RP (2007) Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J Appl Phys 101:064307
Elias M, Amin MK, Firoz SH (2017) Microwave-assisted synthesis of Ce-doped ZnO/CNT composite with enhanced photo-catalytic activity. Ceram Int 43:84–91
Faria B, Guarda C, Silvestre N (2018) Strength and failure mechanisms of CNT-reinforced copper nanocomposite. Compos Part B 145:108–120
Feng L, Lee SH, Wang HL (2015) Synthesis and densification of nano-crystalline hafnium carbide powder. J Eur Ceram Soc 35:4073–4081
Gao LB, Diwu JT, Zhang Q (2015) A green and facile synthesis of carbon-incorporated Co3O4 nanoparticles and their photocatalytic activity for hydrogen evolution. J Nanomater:618492. https://doi.org/10.1155/2015/618492
Gillan EG (2000) Synthesis of nitrogen-rich carbon networks form an energetic molecular azide precursor. Chem Mater 12:3906–3912
Gulino G, Vieira R, Amadou J (2005) C2H6 as an active carbon source for a large scale synthesis of carbon nanotubes by chemical vapor deposition. Appl Catal A 279:89–97
Hamid ZA, Azim AA, Mouez FA (2017) Challenges on synthesis of carbon nanotubes from environmentally friendly green oil using pyrolysis technique. J Anal Appl Pyrolysis 26:218–229
Hou GF, Han DC, Ng V (2017) Gas phase pyrolysis synthesis of carbon nanotubes at high temperature. Mater Des 132:112–118
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Khabashesku VN, Zimmerman JL, Margrave JL (2000) Powder synthesis and characterization of amorphous carbon nitride. Chem Mater 12:3264–3270
Kumi DO, Phaahlamohlaka TN, Dlamini MW (2018) Effect of a titania covering on CNTS as support for the Ru catalysed selective CO methanation. Appl Catal B Environ 232:492–500
Lee SH, Choi SY, Kim HD (2013) ZrB2-SiC nano-powder mixture prepared using ZrSi2 and modified spark plasma sintering. J Am Ceram Soc 96:1051–1054
Liu J, Jiang ZW, Yu HO (2011) Catalytic pyrolysis of polypropylene to synthesize carbon nanotubes and hydrogen through a two-stage process. Polym Degrad Stab 96:1711–1719
Lotsch BV, Schnick W (2005) Thermal conversion of guanylurea dicyanamide into graphitic carbon nitride via prototype CNx precursors. Chem Mater 17:3976–3982
Mishra N, Das G, Ansaldo A (2012) Pyrolysis of waste polypropylene for the synthesis of carbon nanotubes. J Anal Appl Pyrolysis 94:91–98
Nikolaev P, Bronikowski MJ, Bradley RK (1999) Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem Phys Lett 313:91–97
Oberlin A, Endo M, Koyama T (1976) Filamentous growth of carbon through benzene decomposition. J Cryst Growth 32:335–349
Radushkevich L, Lukyanovich V (1952) About the structure of carbon formed by thermal decomposition of carbon monoxide on iron substrate. J Phys Chem 26:88–95
Rajasekar K, Thennarasu S, Rajesh R (2013) Preparation of mesoporous TiO2/CNT nanocomposites by synthesis of mesoporous titania via EISA and their photocatalytic degradation under visible light irradiation. Solid State Sci 26:45–52
Riggs JE, Guo ZX, Carroll DL (2000) Strong luminescence of solubilized carbon nanotubes. J Am Chem Soc 122:5879–5800
Saba F, Zhang FM, Sajjadi SA (2016) Pulsed current field assisted surface modification of carbon nanotubes with nanocrystalline titanium carbide. Carbon 101:261–271
Sari A, Bicer A, Ahmed AA (2018) Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage. Sol Energ Mat and Sol C 179:353–361
Tessonnier JP, Su DS (2011) Recent progress on the growth mechanism of carbon nanotubes: a review. Chem Sus Chem 4:824–847
Wang L, Huang Y, Li C (2015) A facile one pot method to synthesize three dimensional grapheme@carbon nanotubes composite as a high efficiency microwave absorber. Phys Chem Chem Phys 17:2228–2234
Xu JL, Qi XS, Hu Q (2016) Fabrication, characterization, purification and photoluminescence properties of carbon nanomaterials over water-soluble alkali salts. Mater Res Bull 74:218–225
Yan SC, Li ZS, Zou ZG (2009) Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 25:10397–10401
Yan YB, Miao JW, Yang ZH (2015) Carbon nanotube catalysts: recent advances insynthesis, characterization and applications. Chem Soc Rev 44:3295–3346
Yoshida H, Takeda S (2014) Elucidation of the origin of grown-in defects in carbon nanotubes. Carbon 70:266–272
Zhang Y, Gong T, Liu WJ (2005) Strong visible light emission from well-aligned multiwalled carbon nanotubes films under infrared laser irradiation. Appl Phys Lett 87:173114–1731-3
Zhao YL, Wang JM, Chen H (2004) Al-substituted-nickel hydroxide prepared by homogeneous precipitation method with urea. Inter J of Hydro Ener 29:889–896
Funding
This work was supported by the National Natural Science Foundation of China (grant number 51464010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, S., Xiang, D., Hu, Y. et al. One-step rapid preparation of CNTs using the spark plasma–assisted pyrolysis of melamine and its photoluminescence properties. J Nanopart Res 21, 150 (2019). https://doi.org/10.1007/s11051-019-4570-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4570-x