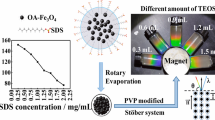
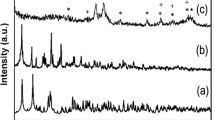
Abstract
We developed a simple and novel approach for the synthesis of Fe3O4@SiO2 nanoparticles with controlled shell thickness, and studied the mechanism. The introduction of N-methyl-2-pyrrolidone (NMP) led to trapping of monomer nuclei in single shell and controlled the shell thickness. Fe3O4@SiO2 controlled the shell thickness, showing a high magnetization value (64.47 emu/g). Our results reveal the role and change in the chemical structure of NMP during the core-shell synthesis process. NMP decomposed to 4-aminobutanoic acid in alkaline condition and decreased the hydrolysis rate of the silica coating process.








Similar content being viewed by others
References
Abbas M, Torati SR, Lee CS, Rinaldi C, Kim CG (2014) Fe3O4/SiO2 core/shell nanocubes: novel coating approach with tunable silica thickness and enhancement in stability and biocompatibility. J Nanomed Nanotechnol 5:244
Dang F, Enomoto N, Hojo J, Enpuku K (2010) Sonochemical coating of magnetite nanoparticles with silica. Ultrason Sonochem 17:193–199
Ding HL, Zhang YX, Wang S, Xu JM, Xu SC, Li GH (2012) Fe3O4@SiO2 core/shell nanoparticles: the silica coating regulations with a single core for different core sizes and shell thicknesses. Chem Mater 24:4572–4580
Jang ES (2012) Preparation of Fe3O4/SiO2 core/shell nanoparticles with ultrathin silica layer. Korean Chem Soc 56:478–483
Kolhatkar AG, Jamison AC, Litvinov D, Willson RC, Lee TR (2013) Tuning the magnetic properties of nanoparticles. Int J Mol Sci 14:15977–16009
Lin L, Zhang S (2012) Effective solvothermal deoxidization of graphene oxide using solid sulphur as a reducing agent. J Mater Chem 22:14385–14393
Lu Y, Yin Y, Mayers BT, Xia Y (2002) Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol−gel approach. Nano Lett 2:183–186
Morel AL, Nikitenko SI et al (2008) Sonochemical approach to the synthesis of Fe3O4@SiO2 core−shell nanoparticles with tunable properties. ACS Nano 2:847–856
Mornet S, Vasseur S, Grasset F, Duguet E (2004) Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem 14:2161–2175
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J D: Appl Phys 36:R167–R181
Philipse AP, MPB v-B, Pathmamanoharan C (1994) Magnetic silica dispersions: preparation and stability of surface-modified silica particles with a magnetic core. Langmuir 10:92–99
Rao AV, Sakhare HM, Tamhankar AK, Shinde ML, Gadave DB, Wagh PB (1999) Influence of N,N-dimethylformamide additive on the physical properties of citric acid catalyzed TEOS silica aerogels. Mater Chem Phys 60:268–273
Safarik I, Safarikova M (2004) Magnetic techniques for the isolation and purification of proteins and peptides. BioMag Res Techn 7:1
Shapiro E, Skrtic S, Koretsky AP (2005) Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med 53:329–338
Singh RK, Kim TH, Patel KD, Knowles JC, Kim HW (2012) Biocompatible magnetite nanoparticles with varying silica-coating layer for use in biomedicine: physicochemical and magnetic properties, and cellular compatibility. J Biomed Mater Res Part A 100A:1734–1742
Tartaj P, Morales MP et al (2003) The preparation of magnetic nanoparticles for applications in biomedicine. Phys D: Appl Phys 36:R182–R197
Wee SB, An GS, Han JS, Oh HC, Choi SC (2016) Co-dispersion behavior and interactions of nano-ZrB2 and nano-SiC in a non-aqueous solvent. Ceram Int 42:4658–4662
Yang C, Wang G, Lu Z, Sun J, Yang W, Zhuang J (2005) Effect of ultrasonic treatment on dispersibility of Fe3O4 nanoparticles and synthesis of multi-core Fe3O4/SiO2 core/shell nanoparticles. J Mater Chem 15:4252–4257
Zhang L, He R, Gu HC (2006) Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci 253:2611–2617
Acknowledgments
The work presented in this paper was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning Science and Technology (2014R1A2A1A11050220).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors S.B. Wee, T.G. Kim, and G.S. An have received research grants from the National Research Foundation of Korea (NRF).
Rights and permissions
About this article
Cite this article
Wee, SB., Oh, HC., Kim, TG. et al. Role of N-methyl-2-pyrrolidone for preparation of Fe3O4@SiO2 controlled the shell thickness. J Nanopart Res 19, 143 (2017). https://doi.org/10.1007/s11051-017-3813-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3813-y