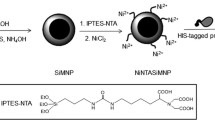
Abstract
Enzyme-catalyzed chemical processes are selective, very productive, and generate little waste. Nevertheless, they may be optimized using enzymes bound to solid supports, which are particularly important for protease-mediated reactions since proteases undergo fast autolysis in solution. Magnetic nanoparticles are suitable supports for this purpose owing to their high specific surface area and to be easily separated from reaction media. Here we describe the immobilization of bovine α-chymotrypsin (αCT) on silica-coated superparamagnetic nanoparticles (Fe3O4@silica) and the characterization of the enzyme-nanoparticle hybrid (Fe3O4@silica-αCT) in terms of protein content, properties, recovery from reaction media, application, and reuse in enzyme-catalyzed peptide synthesis. The results revealed that (i) full acid hydrolysis of the immobilized protease followed by amino acid analysis of the hydrolyzate is a reliable method to determine immobilization yield; (ii) despite showing lower amidase activity and a lower K cat/K m value for a specific substrate than free αCT, the immobilized enzyme is chemically and thermally more stable, magnetically recoverable from reaction media, and can be consecutively reused for ten cycles to catalyze the amide bond hydrolysis and ester hydrolysis of the protected dipeptide Z-Ala-Phe-OMe. Altogether, these properties indicate the potential of Fe3O4@silica-αCT to act as an efficient, suitably stable, and reusable catalyst in amino acid, peptide, and protein chemistry as well as in proteomic studies.






Similar content being viewed by others
References
Aldridge S (2013) Industry backs biocatalysis for greener manufacturing. Nat Biotechnol 31(2):95–96
Arica MY, Bayramoglu G, Biçak N (2004) Characterisation of tyrosinase immobilised onto spacer-arm attached glycidyl methacrylate-based reactive microbeads. Process Biochem 39:2007–2017
Bernal C, Illanes A, Wilson L (2014) Heterofunctional hydrophilic–hydrophobic porous silica as support for multipoint covalent immobilization of lipases: application to lactulose palmitate synthesis. Langmuir 30:3557–3566
Bundy HF (1863) Chymotrypsin-catalyzed hydrolysis of N-acetyl- and N-benzoyl-l-tyrosine p-nitroanilides. Arch Biochem Biophys 102:416–422
Chandrudu S, Simerska P, Toth I (2013) Chemical methods for peptide and protein production. Molecules 18:4373–4388
Chen JP, Su DR (2001) Latex particles with thermo-flocculation and magnetic properties for immobilization of alpha-chymotrypsin. Biotechnol Prog 17:369–375
Christian MS, Brent RL (2001) Teratogen update: evaluation of the reproductive and developmental risks of caffeine. Teratology 64(1):51–78
Clark DS, Bailey JE (2002) Structure-function relationships in immobilized chymotrypsin catalysis. Biotechnol Bioeng 79:539–549
Datta S, Christena LR, Rajaram YSR (2013) Enzyme immobilization: an overview on techniques and support materials. 3 Biotech 3:1–9
El-Ghaffar MAA, Hashem MS (2013) Calcium alginate beads encapsulated PMMA-g-CS nano-particles for α-chymotrypsin immobilization. Carbohydr Polym 92:2095–2102
Faber K (1997) Biotransformations in organic chemistry. Springer, Berlin
Flores-Fernández GM, Griebenow K (2012) Glycosylation improves α-chymotrypsin stability upon encapsulation in poly(lactic-co-glycolic)acid microspheres results. Pharma Sci 2:46–51
Forsberg EM, Green JRA, Brennan JD (2011) Continuous flow immobilized enzyme reactor-tandem mass spectrometry for screening of AChE inhibitors in complex mixtures. Anal Chem 83:5230–5236
Hegedus I, Nagy E (2009) Improvement of chymotrypsin enzyme stability as single enzyme nanoparticles. Chem Eng Sci 64:1053–1060
Homaei AA, Sariri R, Vianello F, Stevanato R (2013) Enzyme immobilization: an update. J Chem Biol 6:185–205
Hong J, Gong PJ, Yu JH, Xua DM, Suna HW, Yao S (2006) Conjugation of α-chymotrypsin on a polymeric hydrophilic nanolayer covering magnetic nanoparticles. J Mol Catal B 42:99–105
Hong J, Gonga P, Xu D, Donga L, Yao S (2007a) Stabilization of α-chymotrypsin by covalent immobilization on amine-functionalized superparamagnetic nanogel. J Biotechnol 128:597–605
Hong J, Xu D, Gong P, Sun H, Dong L, Yao S (2007b) Covalent binding of α-chymotrypsin on the magnetic nanogels covered by amino groups. J Mol Catal B 45:84–90
Hong J, Xu D, Gong P, Yu J, Ma H, Yao S (2008) Covalent-bonded immobilization of enzyme on hydrophilic polymer covering magnetic nanogels. Microporous Mesoporous Mater 109:470–477
Jacinto MJ, Kiyohara PK, Masunaga SH, Jardim RF, Rossi LM (2008) Recoverable rhodium nanoparticles: synthesis, characterization and catalytic performance in hydrogenation reactions. Appl Catal A 338:52–57
Jacinto MJ, Santos OHCF, Jardim RF, Landers R, Rossi LM (2009) Preparation of recoverable Ru catalysts for liquid-phase oxidation and hydrogenation reactions. Appl Catal A 360:177–182
Ju HY, Kuo CH, Too JR, Huanh HY, Twu YK, Chang CMJ, Liu YC, Shieh CJ (2012) Optimal covalent immobilization of α-chymotrypsin on Fe3O4-chitosan nanoparticles. J Mol Catal B 78:9–15
Kaiser E, Colescott RL, Bossinger CD, Cook PI (1970) Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem 34(2):595–598
Kim J, Lee J, Na HB, Kim BC, Youn JK, Kwak JH, Moon K, Lee E, Kim J, Park J, Dohnalkova A, Park HG, Gu MB, Chang H, Grate JW, Hyeon TA (2005) A magnetically separable, highly stable enzyme system based on nanocomposites of enzymes and magnetic nanoparticles shipped in hierarchically ordered, mesocellular, mesoporous silica. Small 12:1203–1207
Leão AMAC, Oliveira EA, Carvalho LB Jr (1991) Immobilization of protein on ferromagnetic dacron. Appl Biochem Biotechnol 31:53–58
Lee J, Na HB, Kim BC, Lee JH, Lee B, Kwak JH, Hwang Y, Park J-G, Gu MB, Kim J, Joo J, Shin C-H, Grate JW, Hyeon T, Kim J (2009) Magnetically-separable and highly-stable enzyme system based on crosslinked enzyme aggregates shipped in magnetite-coated mesoporous silica. J Mater Chem 19:7864–7870
Li DF, Ding HC, Zhou T (2013) Covalent immobilization of mixed proteases, trypsin and chymotrypsin, onto modified polyvinyl chloride microspheres. J Agric Food Chem 61:10447–10453
Liria CW, Romagna CD, Rodovalho NN, Marana SR, Miranda MTM (2008) Dipeptide synthesis in biphasic medium: evaluating the use of commercial porcine pancreatic lipase preparations and the involvement of contaminant proteases. J Braz Chem Soc 19:1574–1581
Lugo-Morales LZ, Loziuk PL, Corder AK, Toups JV, Roberts JG, McCaffrey KA, Sombers LA (2013) Enzyme-modified carbon-fiber microelectrode for the quantification of dynamic fluctuations of nonelectroactive analytes using fast-scan cyclic voltammetry. Anal Chem 85:8780–8786
Machini MT (1985) Termolisina como catalisador na síntese de di-e tripeptídeos contendo asparagina. MSc Thesis, University of São Paulo, São Paulo
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb Technol 26:509–515
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37(4):790–802
Miranda MTM, Tominaga M (1991) Thermolysin as a catalyst in enzymatic synthesis of asparagine-containing peptides II. Int J Pept Protein Res 37(2):128–133
Miranda MTM, Cheng E, Muradian J, Seidel WF, Tominaga M (1986) Thermolysin as a catalyst in enzymatic synthesis of asparagine-containing peptides. Bioorg Chem 14(2):182–193
Narai-Kanayama A, Hanaishi T, Aso K (2012) α-Chymotrypsin-catalyzed synthesis of poly-l-cysteine in a frozen aqueous solution. J Biotechnol 157:428–436
Netto CGCM, Andrade LH, Toma HE (2009) Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron Asymmetry 20:2299–2304
Netto CGCM, Toma HE, Andrade LH (2013) Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J Mol Catal B 85–86:71–92
Palocci C, Chronopoulou L, Venditti I, Cernia E, Diociaiuti M, Fratoddi I, Russo MV (2007) Lipolytic enzymes with improved activity and selectivity upon adsorption on polymeric nanoparticles. Biomacromolecules 8:3047–3053
Pankhurst QA, Connolly J, Jones SK, Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36:167–181
Rossi LM, Quach AD, Rosenzweig Z (2004) Glucose oxidase–magnetite nanoparticle bioconjugate for glucose sensing. Anal Bioanal Chem 380:606–613
Rossi LM, Vono LLR, Silva FP, Kiyohara PK, Duarte EL, Matos JR (2007) A magnetically recoverable scavenger for palladium based on thiol-modified magnetite nanoparticles. Appl Catal A 330:139–144
Rossi LM, Garcia MAS, Vono LLR (2012) Recent advances in the development of magnetically recoverable metal nanoparticle catalysts. J Braz Chem Soc 23:1959–1971
Rossi LM, Costa NJS, Silva FP, Wojcieszak R (2014) Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem 16:2906–2933
Singh RK, Tiwari MK, Singh R, Lee JK (2013) From protein engineering to immobilization: promising strategies for the upgrade of industrial enzymes. Int J Mol Sci 14:1232–1277
Sun J, Hu K, Liu Y, Pan Y, Yang Y (2013) Novel superparamagnetic sanoparticles for trypsin immobilization and the application for efficient proteolysis. J Chromatogr B 942–943:9–14
Taylor I, Howard AG (1993) Measurement of primary amine groups on surface-modified silica and their role in metal binding. Anal Chim Acta 271:77–82
Thomas SM, DiCosmo R, Nagarajan V (2002) Biocatalysis: applications and potentials for the chemical industry. Trends Biotechnol 20(6):238–242
Tsukada H, Blow DM (1985) Structure of α-chymotrypsin refined at 1.68 Å resolution. J Mol Biol 184:703–711
Yamaguchi H, Miyazaki M, Kawazumi H, Maeda H (2010) Multidigestion in continuous flow tandem protease-immobilized microreactors for proteomic analysis. Anal Biochem 407:12–18
Zhang A, Ye F, Lu J, Zhao S (2013) Screening α-glucosidase inhibitor from natural products by capillary electrophoresis with immobilised enzyme onto polymer monolith modified by gold nanoparticles. Food Chem 141:1854–1859
Acknowledgments
This work was supported by grants from FAPESP to MTM (2008/11695-1; 2012/09068-4) and fellowships from CNPq and FAPESP to VAU and NJSC, respectively. We also thank Prof. Dr. Pedro K. Kiyohara (IF-USP) for the TEM images. MTM and LMR are members of the NAPCatSinQ-USP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Carlos Lodeiro Espiño, José Luis Capelo Martinez
This article is part of the topical collection on Composite Nanoparticles
Cleber W. Liria, Vitor A. Ungaro, and Raphaella M. Fernandes have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Liria, C.W., Ungaro, V.A., Fernandes, R.M. et al. Synthesis, properties, and application in peptide chemistry of a magnetically separable and reusable biocatalyst. J Nanopart Res 16, 2612 (2014). https://doi.org/10.1007/s11051-014-2612-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2612-y