Abstract
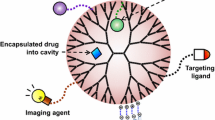
Poly(amidoamine) (PAMAM) dendrimers are a novel class of spherical, well-designed branching polymers with interior cavities and abundant terminal groups on the surface which can form stable complexes with drugs, plasmid DNA, oligonucleotides, and antibodies. Amine‐terminated PAMAM dendrimers are able to solubilize different families of hydrophobic drugs, but the cationic charges on dendrimer surface may disturb the cell membrane. Therefore, surface modification by PEGylation, acetylation, glycosylation, and amino acid functionalization is a convenient strategy to neutralize the peripheral amine groups and improve dendrimer biocompatibility. Anticancer agents can be either encapsulated in or conjugated to dendrimer and be delivered to the tumor via enhanced permeability and retention (EPR) effect of the nanoparticle and/or with the help of a targeting moiety such as antibody, peptides, vitamins, and hormones. Biodegradability, non-toxicity, non-immunogenicity, and multifunctionality of PAMAM dendrimer are the key factors which facilitate steady increase of its application in drug delivery, gene transfection, tumor therapy, and diagnostics applications with precision and selectivity. This review deals with the major topics of PAMAM dendrimers including structure, synthesis, toxicity, surface modification, and also possible new applications of these spherical polymers in biomedical fields as dendrimer-based nanomedicine.



















Similar content being viewed by others
References
Albertazzi L, Gherardini L, Brondi M, Sato SS, Bifone A (2013) In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol Pharm 10:249–260. doi:10.1021/mp300391v
Asthana A, Chauhan AS, Diwan PV, Jain NK (2005) Poly(amidoamine) (PAMAM) dendritic nanostructures for controlled site-specific delivery of acidic anti-inflammatory active ingredient. AAPS PharmSciTech 6:536–542. doi:10.1208/pt060367
Astruc D, Liang L, Rapakousiou A, Ruiz J (2012) Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions a bridge between dendritic architectures and nanomaterials. Acc Chem Res 45:630–640. doi:10.1021/ar200235m
Aulenta F, Hayes W, Rannard S (2003) Dendrimers: a new class of nanoscopic containers and delivery devices. Eur Polym J 39:1741–1771. doi:10.1016/S0014-3057(03)00100-9
Bai CZ, Choi S, Nam K, An S, Park JS (2013) Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int J Pharm 445:79–87. doi:10.1016/j.ijpharm.2013.01.057
Balaji BS, Lewis MR (2009) Double exponential growth of aliphatic polyamide dendrimers via AB2 hypermonomer strategy. Chem Commun 4593–4595. doi:10.1039/B903948A
Baos U, Christensen JB, Heegaard PMH (2006) Dendrimers: design, synthesis and chemical properties. J Mater Chem 16:3785–3798. doi:101039/B611813P
Barrett T, Ravizzini G, Choyke PL, Kobayashi H (2009) Dendrimers application related to bioimaging. IEEE Eng Med Biol Mag 28:12–22. doi:10.1109/MEMB.2008.931012
Barth RF, Adams DM, Soloway AH, Alam F, Darbyt MV (1994) Boronated starburst dendrimer-monoclonal antibody immunoconjugates: evaluation as a potential delivery system for neutron capture therapy. Bioconjug Chem 5:58–66. doi:10.1021/bc00025a008
Beezer AE, King AS, Martin IK, Mitchel JC, Twyman LJ, Wain CF (2003) Dendrimers as potential drug carriers; encapsulation of acidic hydrophobes within water soluble PAMAM derivatives. Tetrahedron 59:3873–3880. doi:10.1016/S0040-4020(03)00437-X
Bhadra D, Bhadra S, Jain S, Jain NK (2003) PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm 257:111–124. doi:10.1016/S0378-5173(03)00132-7
Bielawski K, Bielawska A, Muszyn AM, Poplawska B (2011) Cytotoxic activity of G3 PAMAM-NH2 dendrimer-chlorambucil conjugate in human breast cancer cells. Environ Toxicol Pharmacol 3:364–372. doi:10.1016/j.etap.2011.08.002
Bielinska AU, Kukowska-Latallo JF, Baker JR (1997) The interaction of plasmid DNA with polyamidoamine dendrimers: mechanism of complex formation and analysis of alterations induced in nuclease sensitivity and transcriptional activity of the complexed DNA. Biochim Biophys Acta 1353:180–190. doi:10.1016/S0167-4781(97)00069-9
Bielinska AU, Chen C, Johnson J, Baker JR (1999) DNA complexing with polyamidoamine dendrimers: implications for transfection. Bioconjug Chem 10:843–850. doi:10.1021/bc990036k
Borowska K, Laskowska B, Magon A, Mysliwiec B, Pyda M, Wolowiec S (2010) PAMAM dendrimers as solubilizers and hosts for 8-methoxypsoralene enabling transdermal diffusion of the guest. Int J Pharm 398:185–189. doi:10.1016/j.ijpharm.2010.07.019
Borowska K, Wolowiec S, Rubaj A, Glowniak K, Sieniawska E, Radej S (2012) Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene—in vivo study. Int J Pharm 426:280–283. doi:10.1016/j.ijpharm.2012.01.041
Bosman AW, Janssen HM, Meijer EW (1999) About dendrimers: structure, physical properties, and applications. Chem Rev 99:1665–1688. doi:10.1021/cr970069y
Braun CS, Vetro JA, Tomalia DA, Koe GS, Koe JG, Middaugh CR (2005) Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J Pharm Sci 94:423–436. doi:10.1002/jps.20251
Brothers HM, Piehler LT, Tomalia DA (1998) Slab-gel and capillary electrophoretic characterization of polyamidoamine dendrimers. J Chromatogr A 814:233–246. doi:10.1016/S0021-9673(98)00419-1
Buczkowski A, Sekowski S, Grala A, Palecz D, Milowski K (2011) Interaction between PAMAM-NH2 G4 dendrimer and 5-fluorouracil in aqueous solution. Int J Pharm 408:266–270. doi:10.1016/j.ijpharm.2011.02.014
Buczkowski A, Urbaniak P, Palcez B (2012) Thermochemical and spectroscopic studies on the supramolecular complex of PAMAM-NH2 G4 dendrimer and 5-fluorouracil in aqueous solution. Int J Pharm 428:178–182. doi:10.1016/j.ijpharm.2012.03.004
Bullen HA, Hemmer R, Haskamp A, Cason C, Wall S, Spaulding R, Rossow B, Hester M, Caroway M, Haik KL (2011) Evaluation of biotinylated PAMAM dendrimer toxicity in models of the blood brain barrier: a biophysical and cellular approach. J Biomater Nanobiotechnol 2:485–493. doi:10.4236/jbnb.2011.225059
Campagna S, Ceroni P, Puntoriero F (2012) Designing dendrimers. John Wiley and Sons, Hoboken
Cason CA, Fabre TA, Buhrlage A, Hail KL, Bullen HA (2012) Low-level detection of poly(amidoamine) PAMAM dendrimers using immunoimaging scanning probe microscopy. Int J Anal Chem 1–8. doi:10.1155/2012/341260
Cevc G, Vierl U (2010) Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release 141:277–299. doi:10.1016/j.jconrel.2009.10.016
Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV (2007a) Folate coupled poly(ethyleneglycol) conjugates of anionic poly(amidoamine) dendrimer for inflammatory tissue specific drug delivery. J Biomed Mater Res A 82:92–103. doi:10.1002/jbm.a.31122
Chandrasekar D, Sistla R, Ahmad FJ, Khar RK, Diwan PV (2007b) The development of folate-PAMAM dendrimer conjugates for targeted delivery of anti-arthritic drugs and their pharmacokinetics and biodistribution in arthritic rats. Biomaterials 28:504–512. doi:10.1016/j.biomaterials.2006.07.046
Charles S, Vasanthan N, Kwon D, Sekosan G, Ghosh S (2012) Surface modification of poly(amidoamine) (PAMAM) dendrimer as antimicrobial agents. Tetrahedron Lett 53:6670–6675. doi:10.1016/j.tetlet.2012.09.098
Chauhan AS, Jain NK, Diwan PV, Khopade AJ (2004) Solubility enhancement of indomethacin with poly(amidoamine) dendrimers and targeting to inflammatory regions of arthritic rats. J Drug Target 12:575–583. doi:10.1080/10611860400010655
Chen W, Tomalia DA, Thomas JL (2000) Unusual pH-dependent polarity changes in PAMAM dendrimers: evidence for pH-responsive conformational changes. Macromolecules 33:9169–9172. doi:10.1021/ma000791p
Chen Y, Wang G, Kong D, Zhang Z, Yang K, Liu R, Zhao W, Xu Y (2013) Double-targeted and double-enhanced suicide gene therapy mediated by generation 5 polyamidoamine dendrimers for prostate cancer. Mol Carcinog 52:237–246. doi:10.1002/mc.21850
Cheng Y (2012) Dendrimer-based drug delivery systems: from theory to practice. John Wiley and sons, Hoboken
Cheng Y, Xu T (2005a) Dendrimers as potential drug carriers part I solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur J Med Chem 40:1188–1192. doi:10.1016/j.ejmech.2005.06.010
Cheng Y, Xu T (2005b) Solubility of nicotinic acid in polyamidoamine dendrimer solutions. Eur J Med Chem 40:1384–1389. doi:10.1016/j.ejmech.2005.08.001
Cheng Y, Xu T, Rongqiang F (2005) Polyamidoamine dendrimers used as solubility enhancers of ketoprofen. Eur J Med Chem 40:1390–1393. doi:10.1016/j.ejmech.2005.08.002
Cheng Y, Xu Z, Ma M, Xu T (2008) Dendrimers as drug carriers: applications in different routes of drug administration. J Pharm Sci 97:123–143. doi:10.1002/jps.21079
Cheng Y, Zhao L, Li Y, Xu T (2011) Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev 40:2673–2703. doi:10.1039/C0CS00097C
Choi JS, Nam K, Park JY, Kim JB, Lee JK, Park JS (2004) Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-argenine. J Control Release 99:445–456. doi:10.1016/j.jconrel.2004.07.027
Choi Y, Thomas T, Kotlyar A, Islam MT, Baker JR (2005) Synthesis and functional evaluation of DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targeting. Chem Biol 12:35–43. doi:10.1016/j.chembiol.2004.10.016
Ciolkowski M, Petersen JF, Ficker M, Janaszewska A, Christensen JB, Klajnert B, Bryszewska M (2012) Surface modification of PAMAM dendrimer improves its biocompability. Nanomed Nanotechnol Biol Med 8:815–817. doi:10.1016/j.nano.2012.03.009
Cloninger MJ (2002) Biological applications of dendrimers. Curr Opin Chem Biol 6:742–748. doi:10.1016/S1367-5931(02)00400-3
Crampton HL, Simanek EE (2007) Dendrimers as drug delivery vehicles: non-covalent interactions of bioactive compounds with dendrimers. Polym Int 2(56):489–496. doi:10.1002/pi.2230
D’ Emanuele A, Jevprasesphant R, Penny J, Attwood D (2004) The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J Control Release 95:447–453. doi:10.1016/j.jconrel.2003.12.006
D’Emanuele A, Attwood D (2005) Dendrimer–drug interactions. Adv Drug Deliv Rev 57:2147–2162. doi:10.1016/j.addr.2005.09.012
Delong R, Stephenson K, Loftus T, Fisher M, Alahari S, Nolting A, Juliano ARL (1997) Characterization of complexes of oligonucleotides with polyamidoamine starburst dendrimers and effects on intracellular delivery. J Pharm Sci 86:762–764. doi:10.1021/js960409f
Devarakonda B, Villiers MM (2005) Effect of polyamidoamine (PAMAM) dendrimers on the in vitro release of water-insoluble nifedipine from aqueous gels. AAPS PharmSciTech 6:504–512. doi:10.1208/pt060363
Devarakonda B, Hill RA, De Villiers MM (2004) The effect of PAMAM dendrimer generation size and surface functional group on the aqueous solubility of nifedipine. Int J Pharm 284:133–140. doi:10.1016/j.ijpharm.2004.07.006
Devarakonda B, Hill RA, Liebenberg W, Brits M, Villiers MM (2005) Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int J Pharm 304:193–209. doi:10.1016/j.ijpharm.2005.07.023
Devarakonda B, Otto DP, Judefeind A, Hill RA, Villiers MM (2007) Effect of pH on the solubility and release of furosemide from polyamidoamine (PAMAM) dendrimer complexes. Int J Pharm 345:142–153. doi:10.1016/j.ijpharm.2007.05.039
Dobrovolskaia MA, Patri AK, Simak J, Hall JB, Semberova J, Lacerda SHP, McNeil SE (2012) Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol Pharm 9:382–393. doi:10.1021/mp200463e
Doshi M (2011) Dendrimer and its application. Int J Pharm Sci Rev Res 7:104–111
Duan S, Kouketsu T, Kazama S, Yamada K (2006) Development of PAMAM dendrimer composite membranes for CO2 separation. J Membr Sci 283:2–6. doi:10.1016/j.memsci.2006.06.026
Duncan R, Izzo L (2005) Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev 57:2215–2237. doi:10.1016/j.addr.2005.09.019
Eichman JD, Bielinska AU, Kukowska-Latallo JF, Baker JR (2000) The use of PAMAM dendrimers in the efficient transfer of genetic material into cells. PSTT 3:232–245. doi:10.1016/S1461-5347(00)00273-X
Esfand R, Tomalia DA (2001) Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. DDT 6:427–436. doi:10.1016/S1359-6446(01)01757-3
Filipowicz A, Wolowiec S (2011) Solubility and in vitro transdermal diffusion of riboflavin assisted by PAMAM dendrimers. Int J Pharm 408:152–156. doi:10.1016/j.ijpharm.2011.01.033
Filipowicz A, Wolowiec S (2012) Bioconjugates of PAMAM dendrimers with trans-retinal, pyridoxal, and pyridoxal phosphate. Int J Nanomed 7:4819–4828. doi:10.2147/IJN.S34175
Gautam SP, Verma A (2012) PAMAM dendrimers: novel polymeric nanoarchitectures for solubility enhancement of candesartan cilexetil, research gate. Pharm Sci 1:1–4
Gillies ER, Frechet JMJ (2005) Dendrimers and dendritic polymers in drug delivery. DDT 10:35–43. doi:10.1016/S1359-6446(04)03276-3
Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM (2006) Activity of dendrimer–methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug Chem 17:275–283. doi:10.1021/bc0501855
Haba Y, Kojima C, Harada A, Ura T, Horinaka H, Kono K (2007) Preparation of poly(ethylene glycol)-modified poly(amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability. Langmuir 23:5243–5246. doi:10.1021/la0700826
Haensler J, Szoka FC (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem 4:372–379. doi:10.1021/bc00023a012
Han SC, Kim JH, Lee JW (2012) Convergent synthesis of PAMAM dendrimers containing tetra(ethyleneoxide) at core using click chemistry. Bull Korean Chem Soc 33:3501–3504. doi:10.5012/bkcs.2012.33.10.3501
Hawker CJ, Frkchet JMJ (1990) Preparation of polymers with controlled molecular architecture a new convergent approach to dendritic macromolecules. J Am Chem Soc 112:7638–7647. doi:10.1021/ja00177a027
Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, Holl MMB (2004) Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug Chem 15:774–782. doi:10.1021/bc049962b
Horiguchi Y, Kudo S, Nagasaki Y (2011) Gd@C82 metallofullerenes for neutron capture therapy—fullerene solubilization by poly(ethylene glycol)-block-poly(2-(N, N-diethylamino)ethyl methacrylate) and resultant efficacy in vitro. Sci Technol Adv Mater 12:1–7. doi:10.1021/cr300297r
Hu J, Cheng Y, Wu Q, Zhao L, Xu T (2009) Host-guest chemistry of dendrimer-drug complexes 2 effects of molecular properties of guests and surface functionalities of dendrimers. J Phys Chem B 113:10650–10659. doi:10.1021/jp9047055
Huang RQ, Qu YH, Ke WL, Zhu JH, Pei YY, Jiang C (2007) Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J 21:1117–1125. doi:10.1096/fj.06-7380com
Ina M (2011) Dendrimer: a novel drug delivery system. J Drug Deliv Therap 1:70–74
Inoue K (2000) Functional dendrimers, hyperbranched and star polymers. Prog Polym Sci 25:453–571. doi:10.1016/S0079-6700(00)00011-3
Jain K, Kesharwani P, Gupta U, Jain NK (2010) Dendrimer toxicity: let’s meet the challenge. Int J Pharm 394:122–142. doi:10.1016/j.ijpharm.2010.04.027
Jana S, Gandhi A, Sen KK, Basu SK (2012) Dendrimers: synthesis, properties, biomedical and drug delivery applications. Am J Pharm Tech Res 2:32–55
Janaszewska A, Ciolkowski M, Wrobel D, Petersen JF, Ficker M, Christensen JB, Bryszewska M, Klajnert B (2013) Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomed Nanotechnol Biol Med 9:461–464. doi:10.1016/j.nano.2013.01.010
Jang WD, Nakagishi Y, Nishiyama N, Kawauchi S, Morimoto Y, Kikuchi M, Kataoka K (2006) Polyion complex micelles for photodynamic therapy: incorporation of dendritic photosensitizer excitable at long wavelength relevant to improved tissue-penetrating property. J Control Release 113:73–79. doi:10.1016/j.jconrel.2006.03.009
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A (2003) The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm 252:263–266. doi:10.1016/S0378-5173(02)00623-3
Jia L, Xu JP, Wang H, Ji J (2011) Polyamidoamine dendrimers surface-engineered with biomimetic phosphorylcholine as potential drug delivery carriers. Colloids Surf B 84:49–54. doi:10.1016/j.colsurfb.2010.12.012
Jin GW, Koo H, Nam K, Kim H, Lee S, Park JS, Lee Y (2011) PAMAM dendrimer with a 1,2-diaminoethane surface facilitates endosomal escape for enhanced pDNA delivery. Polymer 52:339–346. doi:10.1016/j.polymer.2010.10.066
Joo WJ, Choi TL, Lee SK, Chung Y, Jung MS, Kim JM (2006) Electronically controlled nonvolatile memory device using PAMAM dendrimer. Org Electron 7:600–606. doi:10.1016/j.orgel.2006.10.001
Khandare J, Kolhe P, Pillai O, Kannan S, Lieh-Lai M, Kanna RM (2005) Synthesis, cellular transport, and activity of polyamidoamine dendrimer-methylprednisolone conjugates. Bioconjug Chem 16:330–337. doi:10.1021/bc0498018
Kim Y, Zimmerman SC (1998) Applications of dendrimers in bio-organic chemistry. Curr Opin Chem Biol 2:733–742. doi:10.1016/S1367-5931(98)80111-7
Kitchens KM, Kolhatkar RB, Swaan P, Ghandehari H (2008) Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol Pharm 5:364–369. doi:10.1021/mp700089s
Kobayashi H, Kawamoto S, Jo SK, Bryant HL, Brechbiel MW, Star RA (2003) Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes and cores. Bioconjug Chem 14:388–394. doi:10.1021/bc025633c
Kobayashi H, Kawamoto S, Bernardo M, Brechbiel MW, Knopp MV, Choyke PL (2006) Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release 111:343–351. doi:10.1016/j.jconrel.2005.12.019
Koc FE, Senel M (2013) Solubility enhancement of non-steroidal anti-inflammatory drugs (NSAIDs) using polypolypropylene oxide core PAMAM dendrimers. Int J Pharm 451:18–22. doi:10.1016/j.ijpharm.2013.04.062
Kofoed J, Reymond JL (2005) Dendrimers as artificial enzymes. Curr Opin Chem Biol 9:656–664. doi:10.1016/j.cbpa.2005.10.013
Kojima C, Kono K, Maruyama K, Takagishi T (2000) Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug Chem 11:910–917. doi:10.1021/bc0000583
Kojima C, Toi Y, Harada A, Kono K (2007) Preparation of poly(ethylene glycol)-attached dendrimers encapsulating photosensitizers for application to photodynamic therapy. Bioconjug Chem 18:663–670. doi:10.1021/bc060244u
Kojima C, Turkbey B, Ogawa M, Bernardo M, Regino CAS, Bryant H, Choyke PL, Kono K, Kobayashi H (2011) Dendrimer-based MRI contrast agents: the effects of PEGylation on relaxivity and pharmacokinetics. Nanomed Nanotechnol Biol Med 7:1001–1008. doi:10.1016/j.nano.2011.03.007
Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H (2007) Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug Chem 18:2054–2060. doi:10.1021/bc0603889
Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M (2003) Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharm 259:143–160. doi:10.1016/S0378-5173(03)00225-4
Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM (2006) Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials 27:660–669. doi:10.1016/j.biomaterials.2005.06.007
Kono K, Fukui T, Takagishi T, Sakurai S, Kojima C (2008a) Preparation of poly(ethylene glycol)-modified poly(amidoamine) dendrimers with a shell of hydrophobic amino acid residues and their function as a nanocontainer. Polymer 49:2832–2838. doi:10.1016/j.polymer.2008.04.048
Kono K, Kojima C, Hayashi N, Nishisaka E, Kiura K, Watarai S, Harada A (2008b) Preparation and cytotoxic activity of poly(ethylene glycol)-modified poly(amidoamine) dendrimers bearing adriamycin. Biomaterials 29:1664–1675. doi:10.1016/j.biomaterials.2007.12.017
Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR (1996) Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci USA 93:4897–4902. doi:10.2307/38873
Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR (2005) Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res 65:5317–5324. doi:10.1158/0008-5472.CAN-04-3921
Kumar A, Yellepeddi VK, Davies GE, Strychar KB, Palakurthi S (2010a) Enhanced gene transfection efficiency by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. Int J Pharm 392:294–303. doi:10.1016/j.ijpharm.2010.03.054
Kumar P, Meena KP, Kumar P, Choudhary C, Thakur DS, Bajpayee P (2010b) Dendrimer: a novel polymer for drug delivery. JITPS 1:252–269
Kumar PD, Kumar PV, Selvam TP, Rao S (2013a) Prolonged drug delivery system of PEGylated PAMAM dendrimers with a anti-HIV drug. Res Pharm 3:8–17
Kumar PD, Kumar PV, Selvam TS, Rao S (2013b) PEG conjugated PAMAM dendrimers with a anti-HIV drug stavudine for prolong release. Res Biotechnol 4:10–18
Kurtoglu YE, Mishra MK, Kannan S, Kannan RM (2010) Drug release characteristics of PAMAM dendrimer–drug conjugates with different linkers. Int J Pharm 384:189–194. doi:10.1016/j.ijpharm.2009.10.017
Labieniec M, Gabryelak T (2008) Preliminary biological evaluation of poli(amidoamine) (PAMAM) dendrimer G3.5 on selected parameters of rat liver mitochondria. Mitochondrion 8:305–312
Labieniec M, Watala C (2009) PAMAM dendrimers—diverse biomedical applications facts and unresolved questions. Cent Eur J Biol 4:434–451. doi:10.2478/s11535-009-0056-7
Lee JW, Kim BK, Kim HJ, Han SC, Shin WS, Jin SH (2006a) Convergent synthesis of symmetrical and unsymmetrical PAMAM dendrimers. Macromolecules 39:2418–2422. doi:10.1021/ma052526f
Lee JW, Kim JH, Kim BK, Kim JH, Shin WS, Jin SH (2006b) Convergent synthesis of PAMAM dendrimers using click chemistry of azide-functionalized PAMAM dendrons. Tetrahedron 62:9193–9200. doi:10.1016/j.tet.2006.07.030
Lee JW, Kim JH, Kim HJ, Han SC, Kim JH, Shin WS, Jin SJ (2007) Synthesis of symmetrical and unsymmetrical PAMAM dendrimers by fusion between azide- and alkyne-functionalized PAMAM dendrons. Bioconjug Chem 18:579–584. doi:10.1021/bc060256f
Lee JW, Han SC, Yun SH, Jin SH (2013) Convergent synthesis of carbazole core PAMAM dendrimer via click chemistry. Bull Korean Chem Soc 34:971–974. doi:10.5012/bkcs.2013.34.3.971
Liu M, Frechet JMJ (1999) Designing dendrimers for drug delivery. PSTT 2:393–401. doi:10.1016/S1461-5347(99)00203-5
Luo D, Haverstick K, Belcheva N, Han E, Saltzman M (2002) Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules 35:3456–3462. doi:10.1021/ma0106346
Ma K, Hu MX, Qi Y, Zou JH, Qiu LY, Jin Y, Ying XY, Sun HY (2009) PAMAM–triamcinolone acetonide conjugate as a nucleus-targeting gene carrier for enhanced transfer activity. Biomaterials 30:6109–6118. doi:10.1016/j.biomaterials.2009.07.036
Maiti PK, Cagin T, Wang G, Goddard WA (2004) Structure of PAMAM dendrimers: generations 1 through 11. Macromolecules 37:6236–6254. doi:10.1021/ma035629b
Maiti PK, Cuang T, Lin ST, Goddard WA (2005) Effect of solvent and pH on the structure of PAMAM dendrimers. Macromolecules 38:979–991. doi:10.1021/ma049168l
Majoros IJ, Keszler B, Woehler S, Bull T, Baker JR (2003) Acetylation of poly(amidoamine) dendrimers. Macromolecules 36:5526–5529. doi:10.1021/ma021540e
Malik N, Evagorou EG, Duncan R (1999) Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs 10:767–776
Malik A, Chaudhary S, Garg G, Tomar A (2012) Dendrimers: a tool for drug delivery. Adv Biol Res 6:165–169. doi:10.5829/idosi.abr.2012.6.4.6254
Margerum LD, Campion BK, Koob M, Shargill N, Laib JJ, Marumoto A, Sontumd PC (1997) Gadolinium(II1) D03A macrocycles and polyethylene glycol coupled to dendrimers. Effect of molecular weight on physical and biological properties of macromolecular magnetic resonance imaging contrast agents. J Alloy Compd 249:185–190. doi:10.1016/S0925-8388(96)02830-7
Markatou E, Gionis V, Chryssikos GD, Hatziantoniou S, Georgopoulos A, Demetzos C (2007) Molecular interactions between dimethoxycurcumin and Pamam dendrimer carriers. Int J Pharm 339:231–236. doi:10.1016/j.ijpharm.2007.02.037
Markowics M, Szymanski P, Ciszewski M, Klys A, Mikiciuk-olasik E (2012) Evaluation of poly(amidoamine) dendrimers as potential carriers of iminodiacetic derivatives using solubility studies and 2D-NOESY NMR spectroscopy. J Biol Phys 38:637–656. doi:10.1007/s10867-012-9277-5
Martin IK, Twyman LJ (2001) The synthesis of unsymmetrical PAMAM dendrimers using a divergent: divergent approach. Tetrahedron Lett 42:1119–1121. doi:10.1016/S0040-4039(00)02001-3
Medina SH, El-Sayed EH (2009) Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev 109:3147–3157. doi:10.1021/cr900174j
Menjoge AR, Kannan RM, Tomalia DA (2010) Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today 15:171–185. doi:10.1016/j.drudis.2010.01.009
Menjoge AR, Rinderknecht AL, Navath RS, Faridnia M, Kim CJ, Romero R, Miller RK, Kannan RM (2011) Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. J Control Release 150:326–338. doi:10.1016/j.jconrel.2010.11.023
Milhem OM, Myles C, McKeown NB, Attwood D, D’Emanuele A (2000) Polyamidoamine starburst dendrimers as solubility enhancers. Int J Pharm 197:239–241. doi:10.1016/S0378-5173(99)00463-9
Mishra MK, Kotta K, Hali M, Wykes S, Gerard HC, Hudson AP, Whittum-Hudson JA, Kannan RM (2011) PAMAM dendrimer-azithromycin conjugate nanodevices for the treatment of Chlamydia trachomatis infections. Nanomed Nanotechnol Biol Med 7:935–944. doi:10.1016/j.nano.2011.04.008
Morales-Sanfrutos J, Megia-Fernandez A, Hernansez-Mateo F, Giron-Gonzalez MD, Salto-Gonzalez R, Santoyo-Gonzalez F (2011) Alkyl sulfonyl derivatized PAMAM-G2 dendrimers as nonviral gene delivery vectors with improved transfection efficiencies. Org Biomol Chem 9:851–864. doi:10.1039/C0OB00355G
Mukherjee SP, Byrne HJ (2013) Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: experimental observation and numerical simulation. Nanomed Nanotechnol Biol Med 9:202–211. doi:10.1016/j.nano.2012.05.002
Najlah M, D’Emanuele A (2006) Crossing cellular barriers using dendrimer nanotechnologies. Curr Opin Pharmacol 6:522–527. doi:10.1016/j.coph.2006.05.004
Navarro G, Ilarduya CT (2009) Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomed Nanotechnol Biol Med 5:287–297. doi:10.1016/j.nano.2008.12.007
Pan G, Lemmouchi Y, Akala EO, Bakare O (2005) Studies on PEGylated and drug-loaded PAMAM dendrimers. J Bioact Compat Polym 20:113–128. doi:10.1177/0883911505049656
Papagiannaros A, Dimas K, Papaioannou GT, Demetzos C (2005) Doxorubicin–PAMAM dendrimer complex attached to liposomes: cytotoxic studies against human cancer cell lines. Int J Pharm 302:29–38. doi:10.1016/j.ijpharm.2005.05.039
Patel HN, Patel PM (2013) Dendrimer applications—a review. Int J Pharm Bio Sci 4:454–463
Patel J, Garala K, Basu B, Ravel M, Dharamsi A (2011) Solubility of aceclofenac in polyamidoamine dendrimer solutions. Int J Pharm Investig 1:135–138. doi:10.4103/2230-973X.85962
Patri AK, Majoros IJ, Baker JR (2002) Dendritic polymer macromolecular carriers for drug delivery. Curr Opin Chem Biol 6:466–471. doi:10.1016/S1367-5931(02)00347-2
Patri AK, Kikowska-Latallo JF, Baker JR (2005) Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev 57:2203–2214. doi:10.1016/j.addr.2005.09.014
Peterson J, Ebber A, Allikmaa V, Lopp M (2001) Synthesis and CZE analysis of PAMAM dendrimers with an ethylenediamine core. Proc Estonian Acad Sci Chem 50:156–166
Pittelkow M, Cheistensen JB (2005) Convergent synthesis of internally branched PAMAM dendrimers. Org Lett 7:1295–1298. doi:10.1021/ol050040d
Prajapat RP, Soni B, Jain S, Bhandari A (2011) Dendrimer: a polymer of 21st century. WebmedCentral Pharm Sci 1:1–15
Prasannal PR, Selvaman P, Gomathi E (2013) Waste water treatment through dendrimer—conjugated magnetic nanoparticles. Int J ChemTech Res 5:1239–1245
Qi R, Gao Y, Tang Y, He RR, Liu TL, Sun S, Li BY, Li YB, Liu G (2009) PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J 11:395–405. doi:10.1208/s12248-009-9116-1
Raghu SC, Berchmans S, Phani KP, Yegnaraman V (2007) PAMAM dendrimers as anchors for the preparation of electrocatalytically active ultrathin metallic films. Chem Asian J 2:775–781. doi:10.1002/asia.200700013
Sadekar S, Ghandehari H (2012) Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev 64:571–588. doi:10.1016/j.addr.2011.09.010
Santos JL, Pandita D, Rodrigues J, Pego AP, Granja PL, Balian G, Tomas H (2010) Receptor-mediated gene delivery using PAMAM dendrimers conjugated with peptides recognized by mesenchymal stem cells. Mol Pharm 7:763–774. doi:10.1021/mp9002877
Saovapakhiran A, D’ Emanuele A, Attwood D, Penny J (2009) Surface modification of PAMAM dendrimers modulates the mechanism of cellular internalization. Bioconjug Chem 20:693–701. doi:10.1021/bc8002343
Sayed-Sweet Y, Hedstrand DM, Spinder R, Tomalia DA (1997) Hydrophobically modified poly(amidoamine) (PAMAM) dendrimers: their properties at the air–water interface and use as nanoscopic container molecules. J Mater Chem 7:1199–1205. doi:10.1039/A700860K
Schcharbin DG, Klajnert B, Bryszewska M (2009) Dendrimers in gene transfection. Biochemistry (Moscow) 74:1070–1079. doi:10.1134/S0006297909100022
Scott RWJ, Wilson OM, Crooks RM (2005) Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J Phys Chem B 109:692–704. doi:10.1021/jp0469665
Sekowski S, Kazmiercakb A, Mazurc J, Przybyszewskad M, Zaborskid M, Schcharbina D, Gabryelak T (2009) The interaction between PAMAM G3.5 dendrimer, Cd2+, dendrimer–Cd2+ complexes and human serum albumin. Colloids Surf B 69:95–98. doi:10.1016/j.colsurfb.2008.11.006
Shao N, Su Y, Hu J, Zhang J, Zhang H, Cheng Y (2011) Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int J Nanomed 6:3361–3372. doi:10.2147/ijn.s27028
Shcharbin D, Mazur J, Szwedzka M, Wasiak M, Palecz B, Przybyszewska M, Zaborski M, Bryszewska M (2007) Interaction between PAMAM 45 dendrimer, cadmium and bovine serum albumin: a study using equilibrium dialysis, isothermal titration calorimetry, zeta-potential and fluorescence. Colloids Surf B 58:286–289. doi:10.1016/j.colsurfb.2007.04.003
Shi X, Majoros IJ, Baker JR (2005) Capillary electrophoresis of poly(amidoamine) dendrimers: from simple derivatives to complex multifunctional medical nanodevices. Mol Pharm 2:278–294. doi:10.1021/mp0500216
Shi X, Wang S, Meshinchi S, Antwerp MEV, Bi X, Lee I, Baker JR (2007) Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small 3:1245–1252. doi:10.1002/smll.200700054
Shieh MJ, Peng CL, Lou PJ, Chiu CH, Tsai TY, Hsu CY, Yeh CY, Lai PS (2008) Non-toxic phototriggered gene transfection by PAMAM-porphyrin conjugates. J Control Release 129:200–206. doi:10.1016/j.jconrel.2008.03.024
Shinde GV, Bangale GS, Umalkar DG, Rathinaraj BS, Yadav CS, Yadav P (2010) Dendrimers. J Pharm Biomed Sci 3:1–8
Shukla S, Wu G, Chatterjee M, Yang W, Sediko M, Diop LA, Mu R, Muller R, Sudimack JJ, Lee RJ, Barth RF, Tjarks W (2003) Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug Chem 14:158–167. doi:10.1021/bc025586o
Singh P, Gupta U, Asthana A, Jain NK (2008) Folate and folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug Chem 19:2239–2252. doi:10.1021/bc800125u
Stojanovic N, Murphy LD, Wagner BD (2010) Fluorescence-based comparative binding studies of the supramolecular host properties of PAMAM dendrimers using anilinonaphthalene sulfonates: unusual host-dependent fluorescence titration behavior. Sensors 10:4053–4070. doi:10.3390/s100404053
Svenson S (2009) Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm 71:445–462. doi:10.1016/j.ejpb.2008.09.023
Svenson S, Tomalia DA (2005) Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev 57:2106–2129. doi:10.1016/j.addr.2005.09.018
Tajabadia M, Khosroshahia ME, Bonakdar S (2013) An efficient method of SPION synthesis coated with third generation PAMAM dendrimer. Colloids Surf A 431:18–26. doi:10.1016/j.colsurfa.2013.04.003
Tao X, Yang YJ, Liu S, Zheng YZ, Fu J, Chen JF (2013) Poly(amidoamine) dendrimer-grafted porous hollow silica nanoparticles for enhanced intracellular photodynamic therapy. Acta Biomater 9:6431–6438. doi:10.1016/j.actbio.2013.01.028
Teow HM, Zhou Z, Najlah M, Yusof SR, Aboott NJ, D’Emanuele A (2013) Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int J Pharm 441:701–711. doi:10.1016/j.ijpharm.2012.10.024
Thomas TP, Choi SK, Li MH, Kotlyar A, Baker JR (2010) Design of riboflavin-presenting PAMAM dendrimers as a new nanoplatform for cancer-targeted delivery. Bioorg Med Chem Lett 20:5191–5194. doi:10.1016/j.bmcl.2010.07.005
Tomalia DA (1994) Starburst/cascade dendrimers: fundamental building blocks for a new nanoscopic chemistry set. Adv Mater 6:529–539. doi:10.1002/adma.19940060703
Tomalia DA (2004) Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic organic chemistry. Aldrichim Acta 37:39–57. doi:10.1016/j.progpolymsci.2005.01.007
Tomalia DA (2009) In quest of a systematic framework for unifying and defining nanoscience. J Nanopart Res 11:1251–1310. doi:10.1007/s11051-009-9632-z
Tomalia DA (2010) Dendrons/dendrimers: quantized, nano-element like building blocks for soft–soft and soft-hard nano-compound synthesis. Soft Matter 6:456–474. doi:10.1039/b917370f
Tomalia DA (2012) Dendritic effects: dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs). New J Chem 36:264–281. doi:10.1039/c1nj20501c
Tomalia DA, Frechet JMJ (2002) Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polym Sci A 40:2719–2728. doi:10.1002/pola.10301
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17:117–132. doi:10.1295/polymj.17.117
Tomalia DA, Naylor AM, Goddard WA (1990) Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl 29:138–175. doi:10.1002/anie.199001381
Tomalia DA, Christensen JB, Baos U (2012) Dendrimers, dendrons, and dendritic polymers: discovery, applications, and the future. Cambridge University Press, New York
Trivedi V, Patel U, Bhimani B, Daslaniya D, Patel G, Vyas B (2012) Dendrimer: polymer of 21st century. IJPRBS 1:1–21
Umeda Y, Kojima C, Harada A, Horinaka H, Kono K (2010) PEG-attached PAMAM dendrimers encapsulating gold nanoparticles: growing gold nanoparticles in the dendrimers for improvement of their photothermal properties. Bioconjug Chem 21:1559–1564. doi:10.1021/bc1001399
Vandamme TF, Brobeck L (2005) Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release 102:23–38. doi:10.1016/j.jconrel.2004.09.015
Venuganti VVK, Perumal OP (2008) Effect of poly(amidoamine) (PAMAM) dendrimer on skin permeation of 5-fluorouracil. Int J Pharm 361:230–238. doi:10.1016/j.ijpharm.2008.05.034
Waite CL, Roth CM (2009) PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjug Chem 20:1908–1916. doi:10.1021/bc900228m
Waite CL, Sparks SM, Uhrich KE, Roth CM (2009) Acetylation of PAMAM dendrimers for cellular delivery of siRNA. BMC Biotechnol 9:1–10. doi:10.1186/1472-6750-9-38
Wang D, Kova PK, Minko T, Nanayakkara V, Kopecek J (2000) Synthesis of starlike N-(2-hydroxypropyl)methacrylamide copolymers: potential drug carriers. Biomacromolecules 1:313–319. doi:10.1021/bm0000236
Wang C, Wyn-Jones E, Sidhu J, Tam KC (2007) Supramolecular complex induced by the binding of sodium dodecyl sulfate to PAMAM dendrimers. Langmuir 23:1635–1639. doi:10.1021/la0625897
Wang W, Xiong W, Wan J, Sun X, Xu H, Yang X (2009a) The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology 20:1–7. doi:10.1088/0957-4484/20/10/105103
Wang Y, Kong W, Song Y, Duan Y, Wang L, Steinhoff G, Kong D, Yu Y (2009b) Polyamidoamine dendrimers with a modified pentaerythritol core having high efficiency and low cytotoxicity as gene carriers. Biomacromolecules 10:617–622. doi:10.1021/bm801333s
Wang B, Navath RS, Menjogo AR, Balakrishnan B, Bellair R, Dai H, Romero R, Kannan S, Kannan RM (2010) Inhibition of bacterial growth and intra amniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int J Pharm 395:298–308. doi:10.1016/j.ijpharm.2010.05.030
Wang H, Shi HB, Yin SK (2011) Polyamidoamine dendrimers as gene delivery carriers in the inner ear: how to improve transfection efficiency (Review). Exp Ther Med 2:777–781. doi:10.3892/etm.2011.296
Wiener EC, Auteri FP, Chen JW, Brechbiel MW, Gansow OA, Schneider DS, Belford RL, Clarkson RB, Lauterbur PC (1996) Molecular dynamics of ion-chelate complexes attached to dendrimers. J Am Chem Soc 118:7774–7782. doi:10.1021/ja9536126
Wiwattanapatapee R, Lomlim L, Saramunee K (2003) Dendrimers conjugates for colonic delivery of 5-aminosalicylic acid. J Control Release 88:1–9. doi:10.1016/S0168-3659(02)00461-3
Wolinsky JB, Grinstaff MW (2008) Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv Drug Deliv Rev 60:1037–1055. doi:10.1016/j.addr.2008.02.012
Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MDJ, Fenstermaker RA (2004) Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug Chem 15:185–194. doi:10.1021/bc0341674
Wu G, Yang W, Barth RF, Kawabata S, Swindall M, Bandyopadhyaya AK, Tjarks W, Khorsandi B, Blue TE, Ferketich AK, Yang M, Christoforidis GA, Sferra TJ, Binns PJ, Riley KJ, Ciesielski MJ, Fenstermaker RA (2007) Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res 13:1260–1268. doi:10.1158/1078-0432.CCR-06-2399
Yang W, Barth RF, Adams DM, Soloway AH (1997) Intratumoral delivery of boronated epidermal growth factor for neutron capture therapy of brain tumors. Cancer Res 57:4333–4339
Yang W, Barth RF, Wu G, Kawabata S, Sferra TJ, Bandyopadhyaya AK, Tjarks W, Ferketich AK, Moeschberger ML, Binns PJ, Riley KJ, Coderre JA, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ (2006) Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res 12:3792–3802. doi:10.1158/10780432.ccr-06-0141
Yang W, Cheng Y, Xu T, Wang X, Wen LP (2009) Targeting cancer cells with biotin-dendrimer conjugates. Eur J Med Chem 44:862–868. doi:10.1016/j.ejmech.2008.04.021
Yellepeddi VK, Kumar A, Palakurthi S (2009) Biotinylated poly(amido)amine (PAMAM) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer Res 29:2933–2944
Yoo H, Juliano RL (2000) Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acid Res 28:4225–4231. doi:10.1093/nar/28.21.4225
Yoo H, Sazani P, Juliano LR (1999) PAMAM dendrimers as delivery agents for antisense oligonucleotides. Pharm Res 16:1799–1804. doi:10.1023/A:1018926605871
Yordanov AT, Lodder AL, Woller EK, Cloninger MJ, Patronas N, Milenic D, Brechbiel MW (2002) Novel iodinated dendritic nanoparticles for computed tomography (CT) imaging. Nano Lett 2:595–599. doi:10.1021/nl020246x
Yu GS, Bae YM, Choi H, Kong B, Choi IS, Choi JS (2011) Synthesis of PAMAM dendrimer derivatives with enhanced buffering capacity and remarkable gene transfection efficiency. Bioconjug Chem 22:1046–1055. doi:10.1021/bc100479t
Zhang XQ, Wang WL, Huang SW, Zhuo RX, Liu ZL, Mao HQ, Leong KW (2005) In vitro gene delivery using polyamidoamine dendrimers with a trimesyl core. Biomacromolecules 6:341–350. doi:10.1021/bm040060n
Zhang XQ, Intra J, Salem AK (2007) Conjugation of polyamidoamine dendrimers on biodegradable microparticles for nonviral gene delivery. Bioconjug Chem 18:2068–2076. doi:10.1021/bc070116l
Zhang Y, Sun Y, Xu X, Zhu H, Huang H, Hunag L, Zhang X, Qi Y, Shen YM (2010) Radiosynthesis and micro-SPECT imaging of 99mTc-dendrimer poly(amido)-amine folic acid conjugate. Bioorg Med Chem Lett 20:927–931. doi:10.1016/j.bmcl.2009.12.075
Zhang TH, Liu CG, Zhang ZQ, Ruan JX (2011) Plasma membrane as the target site of cholic acid analogs. Arch Pharm Res 34:1153–1159. doi:10.1007/s12272-011-0713-z
Zhao L, Cheng Y, Hu J, Wu Q, Xu T (2009) Host-guest chemistry of dendrimer-drug complexes 3 competitive binding of multiple drugs by a single dendrimer for combination therapy. J Phys Chem B 113:14172–14179. doi:10.1021/jp907437e
Zhong H, He ZG, Li Z, Li GY, Shen SR, Li XL (2008) Studies on polyamidoamine dendrimers as efficient gene delivery vector. J Biomater Appl 22:527–544. doi:10.1177/0885328207080005
Zhou J, Wu J, Hafdi N, Behr JP, Erbacherc P, Peng L (2006) PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem Commun 22:2362–2364. doi:10.1039/B601381C
Zhu W, Okollie B, Bhujwalla ZM, Artemov D (2008) PAMAM dendrimer-based contrast agents for MR imaging of Her-2/neu receptors by a three-step pretargeting approach. Magn Reson Med 59:679–685. doi:10.1002/mrm.21508
Zhuo RX, Du B, Lu ZR (1999) In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J Control Release 57:249–257. doi:10.1016/S0168-3659(98)00120-5
Acknowledgments
Financial support for this research was provided by the Scientific and Technical Research Council of Turkey (TÜBİTAK) under PhD fellowship program for foreign citizens grant 2215.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Taghavi Pourianazar, N., Mutlu, P. & Gunduz, U. Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J Nanopart Res 16, 2342 (2014). https://doi.org/10.1007/s11051-014-2342-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2342-1