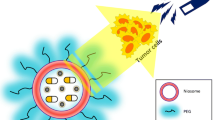
Abstract
One of the main problems of current cancer chemotherapy is the lack of selectivity of anti-cancer drugs to tumor cells, which leads to systemic toxicity and adverse side effects. In order to overcome these limitations, researches on controlled drug delivery systems have gained much attention. Nanoscale-based drug delivery systems provide tumor targeting. Among many types of nanocarriers, superparamagnetic nanoparticles with their biocompatible polymer coatings can be targeted to an intented site by an external magnetic field. Thus, the drug can be carried to the targeted site safely. The aim of this study is to prepare poly(dl-lactic-co-glycolic acid) (PLGA)-coated magnetic nanoparticles and load anti-cancer drug, doxorubicin to them. For this purpose, magnetite (Fe3O4) iron oxide nanoparticles were synthesized as a magnetic core material (MNP) and then coated with oleic acid. Oleic acid-coated MNP (OA-MNP) was encapsulated into PLGA. Effects of different OA-MNP/PLGA ratios on magnetite entrapment efficiency were investigated. Doxorubicin-loaded magnetic polymeric nanoparticles (DOX-PLGA-MNP) were prepared. After the characterization of prepared nanoparticles, their cytotoxic effects on MCF-7 cell line were studied. PLGA-coated magnetic nanoparticles (PLGA-MNP) had a proper size and superparamagnetic character. The highest magnetite entrapment efficiency of PLGA-MNP was estimated as 63 % at 1:8 ratio. Cytotoxicity studies of PLGA-MNP did not indicate any notable cell death between the concentration ranges of 2 and 125 μg/ml. Drug loading efficiency was estimated as 32 %, and it was observed that DOX-PLGA-MNP showed significant cytotoxicity on MCF-7 cells compared to PLGA-MNP. The results showed that prepared nanoparticles have desired size and superparamagnetic characteristics without serious toxic effects on cells. These nanoparticles may be suitable for targeted drug delivery applications.













Similar content being viewed by others
References
Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lübbe AS (2000) Locoregional cancer treatment with magnetic drug targeting. Cancer Res 60(23):6641–6648
Andhariya N, Chudasama B, Mehta R, Upadhyay R (2011) Biodegradable thermoresponsive polymeric magnetic nanoparticles: a new drug delivery platform for doxorubicin. J Nanopart Res 13(4):1677–1688
Ankamwar B, Lai T, Huang J, Liu R, Hsiao M, Chen C, Hwu Y (2010) Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 21(7):075102
Arias J, Gallardo V, Gomez-Lopera S, Plaza R, Delgado A (2001) Synthesis and characterization of poly (ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. J Control Release 77(3):309–321
Arruebo M, Fernández-Pacheco R, Ibarra, Santamaría J (2007) Magnetic nanoparticles for drug delivery. Nano Today 2(3):22–32. doi:10.1016/S1748-0132(07)70084-1
Astete CE, Sabliov CM (2006) Synthesis of poly (dl-lactide-co-glycolide) nanoparticles with entrapped magnetite by emulsion evaporation method. Particul Sci Technol 24(3):321–328
Astete CE, Kumar CS, Sabliov CM (2007) Size control of poly (d, l-lactide-co-glycolide) and poly (d, l-lactide-co-glycolide)-magnetite nanoparticles synthesized by emulsion evaporation technique. Colloid Surf A 299(1):209–216
Berry CC, Curtis ASG (2003) Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D 36(13):R198
Bhosle J, Hall G (2009) Principles of cancer treatment by chemotherapy. Surgery (Oxford) 27(4):173–177. doi:10.1016/j.mpsur.2009.01.006
Bisht S, Maitra A (2009) Dextran–doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1(4):415–425. doi:10.1002/wnan.43
Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J (2010) Magnetic nanoparticles and targeted drug delivering. Pharm Res 62(2):144–149. doi:10.1016/j.phrs.2010.01.014
Coates J (2006) Interpretation of ınfrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, New York. doi:10.1002/9780470027318.a5606
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148(2):135–146. doi:10.1016/j.jconrel.2010.08.027
Gupta AK, Wells S (2004) Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. NanoBiosci IEEE Trans 3(1):66–73
Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V (2008) Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials 29(29):4012–4021. doi:10.1016/j.biomaterials.2008.07.004
Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S, Mohapatra S (2010) Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine 6(1):64–69. doi:10.1016/j.nano.2009.04.002
Lan F, Liu K-X, Jiang W, Zeng X-B, Wu Y, Gu Z-W (2011) Facile synthesis of monodisperse superparamagnetic Fe3O4/PMMA composite nanospheres with high magnetization. Nanotechnology 22(22):225604
Lee S-J, Jeong J-R, Shin S-C, Kim J-C, Chang Y-H, Lee K-H, Kim J-D (2005) Magnetic enhancement of iron oxide nanoparticles encapsulated with poly (d, l-latide-co-glycolide). Colloids Surf A 255(1):19–25
Leonard RCF, Williams S, Tulpule A, Levine AM, Oliveros S (2009) Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (Myocet™). Breast 18(4):218–224. doi:10.1016/j.breast.2009.05.004
Liu X, Kaminski MD, Guan Y, Chen H, Liu H, Rosengart AJ (2006) Preparation and characterization of hydrophobic superparamagnetic magnetite gel. J Magn Magn Mater 306(2):248–253
Mahmoudi M, Sant S, Wang B, Laurent S, Sen T (2011) Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev 63(1–2):24–46. doi:10.1016/j.addr.2010.05.006
McBain SC, Yiu HH, Dobson J (2008) Magnetic nanoparticles for gene and drug delivery. Int J Nanomed 3(2):169
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229. doi:10.1124/pr.56.2.6
Mishra B, Patel BB, Tiwari S (2010) Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 6(1):9–24. doi:10.1016/j.nano.2009.04.008
Muthu M (2009) Nanoparticles based on PLGA and its co-polymer: an overview. Asian J Pharm 3(4):266
Okassa LN, Marchais H, Douziech-Eyrolles L, Hervé K, Cohen-Jonathan S, Munnier E, Soucé M, Linassier C, Dubois P, Chourpa I (2007) Optimization of iron oxide nanoparticles encapsulation within poly (d, l-lactide-co-glycolide) sub-micron particles. Eur J Pharm Biopharm 67(1):31–38
Raut S, Kirthivasan B, Bommana M, Squillante E, Sadoqi M (2010) The formulation, characterization and in vivo evaluation of a magnetic carrier for brain delivery of NIR dye. Nanotechnology 21(39):395102
Şen GP (2009) Poly (Dl-Lactic-Co-Glycolic Acid) microparticles and synthetic peptide drug conjugate for anti-cancer drug delivery. MSc Thesis, METU. Retrieved on October 23, 2011, from http://etd.lib.metu.edu.tr/upload/12611405/index.pdf
Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, Douziech-Eyrolles L, Soucé M, Dubois P, Chourpa I (2007) Comparative study of doxorubicin-loaded poly (lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm 66(3):488–492
Tural B, Özkan N, Volkan M (2009) Preparation and characterization of polymer coated superparamagnetic magnetite nanoparticle agglomerates. J Phys Chem Solids 70(5):860–866
Veiseh O, Gunn JW, Zhang M (2010) Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62(3):284–304. doi:10.1016/j.addr.2009.11.002
Wassel RA, Grady B, Kopke RD, Dormer KJ (2007) Dispersion of super paramagnetic iron oxide nanoparticles in poly (d, l-lactide-co-glycolide) microparticles. Colloids Surf A 292(2):125–130
Widder KJ, Senyel AE, Scarpelli GD (1978) Magnetic microspheres: a model system of site specific drug delivery in vivo. Proceedings of the society for experimental biology and medicine society for experimental biology and medicine (New York, NY) 158(2):141–146
Yang J, Park SB, Yoon H-G, Huh YM, Haam S (2006) Preparation of poly ε-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int J Pharm 324(2):185–190. doi:10.1016/j.ijpharm.2006.06.029
Yang K, Peng H, Wen Y, Li N (2010) Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl Surf Sci 256(10):3093–3097
Zhang Y, Chatterjee DK (2007) Liposomes, dendrimers and other polymeric nanoparticles for targeted delivery of anticancer agents—a comparative study. In: Nanotechnologies for the life sciences. Wiley-VCH Verlag GmbH & Co. KGaA, New York. doi:10.1002/9783527610419.ntls0069
Zhang L, He R, Gu H-C (2006) Oleic acid coating on the monodisperse magnetite nanoparticles. Appl Surf Sci 253(5):2611–2617. doi:10.1016/j.apsusc.2006.05.023
Acknowledgments
This study was supported by the Research Funds of Middle East Technical University Grant No: BAP-07-02-2010-00-01 and The Scientific and Technical Research Council of Turkey (TUBITAK) Grant No: 109T949. Prof. Dr. Güngör Gündüz is acknowledged for his valuable comments on this research. Assoc.Prof.Dr. Bora Maviş is acknowledged for his support in FTIR Spectroscopy measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tansık, G., Yakar, A. & Gündüz, U. Tailoring magnetic PLGA nanoparticles suitable for doxorubicin delivery. J Nanopart Res 16, 2171 (2014). https://doi.org/10.1007/s11051-013-2171-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2171-7