Abstract
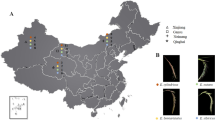
Tall fescue is a perennial cool-season grass with economic importance especially in temperate regions of the northern hemisphere. This study was done to assess the genetic diversity and population structure of 90 tall fescue populations and cultivars using ISSR and EST-SSR markers in order to categorize valuable populations for breeding programs and to construct the core collection of tall fescue collection in Iran. The 10 EST-SSR primer pairs amplified 92 alleles. The allele numbers varied from 4 to 13 alleles per locus with an average of 9.2 alleles, of which 84 (90.6%) were polymorphic with an average of 8.4 polymorphic bands per primer. The 39 ISSR primers totally produced 387 scorable bands, of which 335 (86.6%) were polymorphic with an average of 8.6 polymorphic bands per primer. The amplified markers by ISSR primers varied from 6 to 14 markers per primer with an average of 9.92 markers per primer. The 90 tall fescue populations using both EST-SSR and ISSR data were classified into two clusters by UPGMA method that was coincide with PCA and structure analysis results. The turf-type and forage-type populations were clearly separated. Based on the results, the Iranian populations provide a valuable and novel germplasm to employ in tall fescue varietal improvement programs for both forage and turf-type applications. This progression is an important step to introduce this collection for development of a core collection of tall fescue germplasm in Iran.







Similar content being viewed by others
Abbreviations
- ISSR:
-
Inter-simple sequence repeat
- PCR:
-
Polymerase chain reaction
- EST-SSR:
-
Expressed sequence tag-simple sequence repeat
- UPGMA:
-
Unweighted pair group method with arithmetic mean
- PCA:
-
Principal component analysis
- PIC:
-
Polymorphic information content
- He:
-
Expected heterozygosity
- Ne:
-
Number of effective alleles
- I:
-
Shannon’s information index
- PPL:
-
Percentage of polymorphic loci
References
Jenkins TJ (1954) Interspecific and intergeneric hybrids in herbage grasses. J Genet 28:252–281. https://doi.org/10.1007/BF02981525
Thomas HM, Morgan WG, Humphreys MW (2003) Designing grasses with a future combining the attributes of Lolium and Festuca. Euphytica 133:19–26. https://doi.org/10.1023/A:1025694819031
Spangenberg PG, Wang ZY, Wu X, Nage VA, Iglesias L, Potrykus I (1995) Transgenic tall fescue (Festuca arundinacea) and red fescue (F. rubra) plants from microprojectile bombardment of embryogenic suspension cells. J Plant Physiol 145:693–701. https://doi.org/10.1016/S0176-1617(11)81283-6
Saikkonen K, Phillips TD, Faeth SH, McCulley RL, Saloniemi I, Helander M (2016) Performance of endophyte infected tall fescue in Europe and North America. PLoS ONE 10:1–18. https://doi.org/10.1371/journal.pone.0157382
Fu K, Zhihui G, Xinquan Z, Yan F, Wendan W, Daxu L, Yan P, Linkai H, Ming S, Shiqie B, Xiao M (2016) Insight into the genetic variability analysis and cultivar identification of tall fescue by using SSR markers. Hereditas 153:9–20. https://doi.org/10.1186/s41065-016-0013-1
Talukder SK, Saha MC (2017) Toward genomics-based breeding in C3 cool-season perennial grasses. Front Plant Sci 8:1317. https://doi.org/10.3389/fpls.2017.01317
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237. https://doi.org/10.1046/j.1523-1739.2003.01236.x
Spooner DM, Gavrilenko T, Jansky SH, Ovchinnikova A, Krylova E, Knapp S, Simon R (2010) Ecogeography of ploidy variation in cultivated potato (Solanum Sect. Petota). Am J Bot 97:2049–2060. https://doi.org/10.3732/ajb.1000277
Godwin ID, Aitken EA, Smith LW (1997) Application of inter simple sequence repeat (ISSR) markers to plant genetics. Electrophoresis 18:1524–1528. https://doi.org/10.1002/elps.1150180906
Ghorbanzadeh Naghab M, Panahi B (2017) Molecular characterization of Iranian black cumin (Nigella sativa L.) accessions using RAPD marker. Biotechnologia 98:97–102. https://doi.org/10.5114/bta.2017.68308
Mahmoudi B, Panahi B, Mohammadi SA, Daliri M, Babayev MS (2014) Microsatellite based phylogeny and bottleneck studies of Iranian indigenous goat populations. Anim Biotechnol 25:210–222. https://doi.org/10.1080/10495398.2013.850431
Lauvergeat V, Barre P, Bonnet M, Ghesquiere M (2005) Sixty simple sequence repeat markers for use in the Festuca-Lolium complex of grasses. Mol Ecol Notes 5:401–405. https://doi.org/10.1111/j.1471-8286.2005.00941.x
Wei W, Qi X, Wang L, Zhang Y, Hua W, Li D, Haixia L, Zhang X (2011) Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genom 12:1–13. https://doi.org/10.1186/1471-2164-12-451
Fu YB, Peterson GW, Yu JK, Gao L, Jia J, Richards KW (2006) Impact of plant breeding on genetic diversity of the Canadian hard red spring wheat germplasm as revealed by EST-derived SSR markers. Theor Appl Genet 112:1239–1247. https://doi.org/10.1007/s00122-006-0225-2
Nicot N, Chiquet V, Gandon B, Amilhat L, Legeai F, Leroy P, Bernard M, Sourdille P (2004) Study of simple sequence repeat (SSR) markers from wheat expressed sequence tags (ESTs). Theor Appl Genet 109:800–805. https://doi.org/10.1007/s00122-004-1685-x
Holton TA, Christopher JT, McClure L, Harker N, Henry RJ (2002) Identification and mapping of polymorphic SSR markers from expressed gene sequences of barley and wheat. Mol Breed 9:63–71. https://doi.org/10.1023/A:1026785207878
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhoeur S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Srinivas G, Satish K, Madhusudhana R, Seetharama N (2009) Exploration and mapping of microsatellite markers from subtracted drought stress ESTs in Sorghum bicolor (L.) Moench. Theor Appl Genet 118:703–717. https://doi.org/10.1007/s00122-008-0931-z
Kota R, Varshney RK, Thiel T, Dehmer KJ, Graner A (2001) Generation and comparison of EST-derived SSRs and SNPs in barley (Houdeum vulgare L.) Hereditas 135:145–151. https://doi.org/10.1007/s11032-008-9216-0
Thiel T, Michalek W, Varshney RK, Graner A (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet 106:411–422. https://doi.org/10.1007/s00122-002-1031-0
Malay C, Saha MC, John D, Cooper MA (2006) Tall fescue genomic SSR markers: development and transferability across multiple grass species. Theor Appl Genet 113:1449–1458. https://doi.org/10.1007/s00122-006-0391-2
Amini F, Mirlohi AF, Majidi MM, Shojaiefar S, Kolliker R (2011) Improved polycross breeding of tall fescue through marker-based parental selection. Plant Breed 130:701–707. https://doi.org/10.1111/j.1439-0523.2011.01884.x
Sharifi-Tehrani M, Mardi M, Sahebi P, Catalán P, Díaz-Pérez A (2009) Genetic diversity and structure among Iranian tall fescue populations based on genomic-SSR and EST-SSR marker analysis. Plant Syst Evol 282:57–70. https://doi.org/10.1007/s00606-009-0207-3
Salehi M, Salehi H, Niazi A, Ghobadi C (2014) Convergence of goals: phylogenetical, morphological, and physiological characterization of tolerance to drought stress in tall fescue (Festuca arundinacea Schreb). Mol Biotechnol 56:248–257. https://doi.org/10.1007/s12033-013-9703-3
Panahi B, Afza R, Ghorbanzadeh Neghab M, Mahmoodnia M, Paymard B (2013) Relationship among AFLP, RAPD marker diversity and Agromorphological traits in safflower (Carthamus tinctorius L.) Prog Mol Biol Transl Sci 3:90–99. https://doi.org/10.22059/PBS.2013.32098
Panahi B, Ghorbanzadeh Naghab M (2013) Genetic characterization of Iranian safflower (Carthamus tinctorius) using inter simple sequence repeats (ISSR) markers. Physiol Mol Biol Plants 19:239–243. https://doi.org/10.1007/s12298-012-0155-1
Nie ZN, Chapman DF, Tharmaraj J, Clements R (2004) Effects of pasture species mixture, management, and environment on the productivity and persistence of dairy pastures in south-west Victoria. 2. Plant population density and persistence. Aust J Agric Res 55:637–643. https://doi.org/10.1071/AR03175
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Malay MC, Mian MAR, Zwonitzer JC, Chekhovskiy K, Hopkins AA (2005) An SSR- and AFLP-based genetic linkage map of tall fescue (Festuca arundinacea Schreb.). Theor Appl Genet 110:323–336. https://doi.org/10.1007/s00122-004-1843-1
Sankar AA, Moore GA (2001) Evaluation of inter-simple sequence repeat analysis for mapping in Citrus and extension of genetic linkage map. Theor Appl Genet 102:206–214. https://doi.org/10.1007/s001220051637
Rohlf J (2012) NTSYS-pc: numerical taxonomy and multivariate analysis system, version 2.2, vol 22. Exeter Software, Setauket, New York, pp 14–26. https://doi.org/10.2307/2684761
Roldan-Ruiz I, Dendauw J, Vanbockstaele E, Depicker A, De Loose M (2000) AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp). Mol Breed 6:125–134. https://doi.org/10.1023/A:1009680614564
Pritchard-Novembre J (2016) Pritchard, Stephens, and Donnelly on population structure. Genetics 204:391–393. https://doi.org/10.1534/genetics.116.195164
Awasthi AK, Nagaraja GM, Naik GV, Kanginakudru S (2004) Genetic diversity and relationships in mulberry (genus Morus) as revealed by RAPD and ISSR marker assays. BMC Genet 5:1–9. https://doi.org/10.1186/1471-2156-5-1
Dangi RS, Lagu MD, Choudhary LB, Ranjekar PK, Gupta VS (2004) Assessment of genetic diversity in Trigonella foenum-graecum and Trigonella caerulea using ISSR and RAPD markers. BMC Plant Biol 4:1–11. https://doi.org/10.1186/1471-2229-4-13
Sehgal D, Rajpal VR, Raina SN, Sasanuma T, Sasakuma T (2009) Assaying polymorphism at DNA level for genetic diversity diagnostics of the safflower (Carthamus tinctorius L.) world germplasm resources. Genetica 135:457–470. https://doi.org/10.1007/s10709-008-9292-4
Aggarwal RK, Hendre PS, Varshney RK, Bhat PR, Krishnakumar V, Singh L (2007) Identification, characterization and utilization of EST-derived genic microsatellite markers for genome analyses of coffee and related species. Theor Appl Genet 114:359–372. https://doi.org/10.1007/s00122-006-0440-x
Borna F, Luo S, Ahmad NM, Nazeri V, Shokrpour M, Trethowan R (2017) Genetic diversity in populations of the medicinal plant Leonurus cardiaca L. revealed by inter-primer binding site (iPBS) markers. Genet Resour Crop Evol 3:479–492. https://doi.org/10.1007/s10722-016-0373-4
Pivoriene O, Pasakinskiene I, Brazauskas G, Lideikyte L, Jenen LB, Lubberstedt T (2008) Inter-simple sequence repeat (ISSR) loci mapping in the genome of perennial ryegrass. Agronomy 54:17–21. https://doi.org/10.2478/v10054-008-0004-x
Bornet B, Branchard M (2001) Nonanchored inter simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Mol Biol Rep 19:209–215. https://doi.org/10.1007/BF02772892
Wang ML, Barkley NA, Jenkins TM (2009) Microsatellite markers in plants and insects. Part I. Applications of biotechnology. Genes Genomes Genom 3:54–67
Souframanien J, Gopalakrishna T (2004) A comparative analysis of genetic diversity in blackgram genotypes using RAPD and ISSR markers. Theor Appl Genet 109:1687–1693. https://doi.org/10.1007/s00122-004-1797-3
Belaj A, Satovic Z, Cipriani G, Baldoni L, Testolin R, Rallo L, Trujillo I (2003) Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effectiveness in establishing genetic relationships in olive. Theor Appl Genet 107:736–744. https://doi.org/10.1007/s00122-003-1301-5
Dreisigacker S, Zhang P, Warburton ML, Skovmand D, Hoisington D, Melchinger AE (2005) Genetic diversity among and within CIMMYT wheat landrace accessions investigated with SSRs and implications for plant genetic resources management. Crop Sci 45:653–661. https://doi.org/10.2135/cropsci2005.0653
Kolliker R, Herrmann D, Boller B, Widmer F (2003) Swiss Mattenklee landraces, a distinct and diverse genetic resource of red clover (Trifolium pratense L.) Theor Appl Genet 107:306–315. https://doi.org/10.1007/s00122-003-1248-6
Semagn K, Bjørnstad A, Ndjiondjop MN (2006) Review an overview of molecular marker methods for plants. Afr J Plant Sci Biotechnol 25:2540–2568
Ekenel HK, Sankur B (2004) Feature selection in the independent component subspace for face recognition. Pattern Recognit Lett 25:1377–1388. https://doi.org/10.1016/j.patrec.2004.05.013
Zhang J, Wu D, Wang C, Qu H, Zou X, Yang C (2007) Genetic diversity analysis of Quercus mongolica populations with inter-simple sequence repeats (ISSR). JBES Sci 15:292–299
Lal RK, Gupta P, Sarkar S (2018) Phylogenetic relationships, path and principal component analysis for genetic variability and high oil yielding clone selection in Vetiver (Vetiveria zizanioides L.) Nash. J Plant Genet Breed 2:1–8
Nagaoka T, Ogihara Y (1997) Applicability of intersimple sequence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theor Appl Genet 94:597–602. https://doi.org/10.1007/s001220050456
Kubik C, Sawkins M, Meyer WA, Gaut BS (2001) Genetic diversity in seven perennial ryegrass (Lolium perenne L.) cultivars based on SSR markers. Crop Sci 41:1565–1572. https://doi.org/10.2135/cropsci2001.4151565x
Madesi P, Abraham EM, Kalivas A, Ganopoulos Ι, Tsaftaris A (2014) Genetic diversity and structure of natural Dactylis glomerata L. populations revealed by morphological and microsatellite-based (SSR/ISSR) markers. Genet Mol Res 13:4226–4240. https://doi.org/10.4238/2014.June.9.8
Hand ML, Cogan NOI, Forster JW (2012) Molecular characterization and interpretation of genetic diversity within globally distributed germplasm collections of tall fescue (Festuca arundinacea Schreb.) and meadow fescue (F. pratensis Huds.) Theor Appl Genet 124:1127–1137. https://doi.org/10.1007/s00122-011-1774-6
Acknowledgements
The financial support of the Agricultural Biotechnology Research Institute of Iran (ABRII) is gratefully acknowledged.
Funding
This work was supported by the Agricultural Biotechnology Research Institute of Iran (ABRII) (Grant Numbers 12-05-05-015-96024-960689).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
No ethical issues were promulgated.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahabzadeh, Z., Mohammadi, R., Darvishzadeh, R. et al. Genetic structure and diversity analysis of tall fescue populations by EST-SSR and ISSR markers. Mol Biol Rep 47, 655–669 (2020). https://doi.org/10.1007/s11033-019-05173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05173-z