Abstract
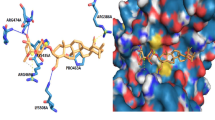
In spite of several studies that have shown the cytotoxic effects of amygdalin on the different cancer cell lines, however, the chemopreventive potential of amygdalin on the breast cancer cell line is not completely understood. We investigated the effect of amygdalin on the cell death and the level of pro-apoptotic Bax protein and anti-apoptotic Bcl-2 protein in SK-BR-3 human breast cancer cell line. The cell viability of SK-BR-3 cells was evaluated by MTT assay in different concentration of amygdalin. The level of Bax and Bcl-2 in SK-BR-3 cells were measured by western blot analysis. For statistical analysis, One-way ANOVA was used for the comparison of Bax and Bcl-2 protein level and percent of cell viability between groups. The molecular docking studies of amygdalin within the Bcl-2 (PDB ID: 4LVT) and HER2 (PDB ID: 3RCD) active site, were performed using AutoDock 4.2.5. Amygdalin induced a significant reduction of cell viability in SK-BR-3 after 24-h treatment in a dose-dependent manner. Also, amygdalin causes an increase in pro-apoptotic Bax protein and a decrease in anti-apoptotic Bcl-2 protein expression in the SK-BR-3 cells. Molecular docking studies showed that amygdalin interacts with the active site amino acids of Bcl-2 and HER2 through hydrogen bonding and some hydrophobic interactions. Amygdalin can induce apoptotic death in SK-BR-3 cells by increasing pro-apoptotic Bax protein and decreasing anti-apoptotic Bcl-2 protein expression. The results suggest that amygdalin may be a valuable candidate for the treatment of breast cancer, especially in HER2 positive cells.




Similar content being viewed by others
References
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28(1):92–98
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Wang J, Jiang Y-F (2012) Natural compounds as anticancer agents: experimental evidence. World J Exp Med 2(3):45–57
Kotecha R, Takami A, Espinoza JL (2016) Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget 7(32):52517–52529
Doughari JH (2012) Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents, in phytochemicals—a global perspective of their role in nutrition and health. InTechOpen
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ (2006) Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3(5):269–280
Ménard S, Tagliabue E, Campiglio M, Pupa SM (2000) Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol 182(2):150–162
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Valabrega G, Montemurro F, Aglietta M (2007) Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 18(6):977–984
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73(8):2013–2026
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J et al (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275(5303):1129–1132
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139(5):1281–1292
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6(2):99–104
Tanaka R, Nitta A, Nagatsu A (2014) Application of a quantitative 1 H-NMR method for the determination of amygdalin in Persicae semen, Armeniacae semen, and Mume fructus. J Nat Med 68(1):225–230
Bolarinwa IF, Orfila C, Morgan MR (2015) Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem 170:437–442
Lee J, Zhang G, Wood E, Rogel Castillo C, Mitchell AE (2013) Quantification of amygdalin in nonbitter, semibitter, and bitter almonds (Prunus dulcis) by UHPLC-(ESI) QqQ MS/MS. J Agric Food Chem 61(32):7754–7759
Cassileth BR, Lucarelli CD (2003) Herb-drug interactions in oncology. PMPH-USA, Raleigh
Moertel CG et al (1982) A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. N Engl J Med 306(4):201–206
Miller KW, Anderson JL, Stoewsand GS (1981) Amygdalin metabolism and effect on reproduction of rats fed apricot kernels. J Toxicol Environ Health 7(3–4):457–467
Blaheta RA, Nelson K, Haferkamp A, Juengel E (2016) Amygdalin, quackery or cure? Phytomedicine 23(4):367–376
Song Z, Xu X (2014) Advanced research on anti-tumor effects of amygdalin. J Cancer Res Ther 10(5):3–7
Lee HM, Moon A (2016) Amygdalin regulates apoptosis and adhesion in Hs578T triple-negative breast cancer cells. Biomol Ther (Seoul) 24(1):62–66
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al (2013) ABT-199, a potent and selective Bcl-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–27891
Moertel CG, Ames MM, Kovach JS, Moyer TP, Rubin JR, Tinker JH (1981) A pharmacologic and toxicological study of amygdalin. JAMA 245(6):591–594
Ghoncheh M, Pournamdar Z, Salehiniya H (2016) Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev 17(S3):43–46
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245
Qian L, Xie B, Wang Y, Qian J (2015) Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int J Clin Exp Pathol 8(5):5363–5370
Chang H-K, Shin M-S, Yang H-Y, Lee J-W, Kim Y-S, Lee M-H et al (2006) Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull 29(8):1597–1602
Makarević J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I et al (2014) Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 9(8):e105590
Juengel E, Thomas A, Rutz J, Makarevic J, Tsaur I, Nelson K et al (2016) Amygdalin inhibits the growth of renal cell carcinoma cells in vitro. Int J Mol Med 37(2):526–532
Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C et al (2013) Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol 35(1):43–51
Giannetti L, Consolo U, Magnoni C, Lo Muzio L (2004) Apoptosis: escaping strategies in human skin cancer. Oncol Rep 11(2):401–405
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13(15):1899–1911
Korsmeyer SJ (1999) Bcl-2 gene family and the regulation of programmed cell death. Cancer Res 59(7 Suppl):1693s–1700s
Dorr RT, Paxinos J (1978) The current status of laetrile. Ann Intern Med 89(3):389–397
Greenberg DM (1980) The case against laetrile: the fraudulent cancer remedy. Cancer 45(4):799–807
Young VR, Newberne PM (1981) Vitamins and cancer prevention: issues and dilemmas. Cancer 47(5 Suppl):1226–1240
Carpenter RL, Lo HW (2013) Regulation of apoptosis by HER2 in breast cancer. J Carcinog Mutagen 2013(Suppl 7):003
Acknowledgements
This paper is issued from the thesis of Bahman Moradipoodeh and financial support was provided by Hyperlipidemia Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant No. HLRC-9602).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradipoodeh, B., Jamalan, M., Zeinali, M. et al. In vitro and in silico anticancer activity of amygdalin on the SK-BR-3 human breast cancer cell line. Mol Biol Rep 46, 6361–6370 (2019). https://doi.org/10.1007/s11033-019-05080-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05080-3