Abstract
The present study details on the mechanism of synthesis of bis (4-formyl-2 methoxy phenyl carbonate), using two green reagents dimethyl carbonate and vanillin for application as therapeutic agent. The synthesized FMPC was identified from the 13C nuclear magnetic resonance spectra. The novel modified Schiff base nanoparticles resulted from the crosslinking of FMPC with chitosan were confirmed by cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance spectroscopy. The incorporation of the FMPC was identified from the amorphous X-ray diffraction patterns of C-FMPC-Nps. The thermal stability of the formed nanoparticles was predicted using thermogravimetric analysis. The morphology of the nanoparticles as observed from HRTEM was found to be smooth and spherical in nature. Both FMPC and C-FMPC-Nps showed significant radical scavenging potential and anticancer property. The carbonate ester backbone and the moiety present in chitosan-FMPC-nanoparticles, underwent hydrolysis at the targeted cancer causing microenvironment to release vanillin and chitosan and enhance the anticancer activity. Both FMPC and C-FMPC-Nps exhibits a dose dependent cytotoxicity towards the different cell lines and it was tested with a commercial drug for application studies.
Graphical abstract
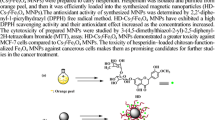
Effective synthesis of FMPC, successful incorporation onto chitosan nanoparticles for the formation of C-FMPC-Nps. The formed Schiff base compound proves to have enhanced antioxidant and anticancer efficacy.




















Similar content being viewed by others
Abbreviations
- FMPC:
-
Bis (4-formyl-2 methoxy phenyl carbonate)
- DMC:
-
Dimethyl carbonate
- 13C-NMR:
-
13C nuclear magnetic resonance
- Nps:
-
Nanoparticles
- C:
-
Chitosan
- CP/MAS:
-
13C-NMR cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance
- XRD:
-
X-ray diffraction
- TGA:
-
Thermogravimetric analysis
- HRTEM:
-
High resolution transmission electron microscopy
- TPP:
-
Sodium tripolyphosphate
- ATR:
-
Attenuated total reflectance
- D2O:
-
Deuterium oxide
- EI-MS:
-
Electron ionization mass spectrometry
- ZrO:
-
Zirconium oxide
- KHZ:
-
Kilohertz
- TMS:
-
Tetramethyl silane
- TEA:
-
Triethyl amine
- MTT:
-
[3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
- DPPH:
-
(1,1-Diphenyl-2-picryl hydrazyl)
- MEM:
-
Minimal essential medium
- FBS:
-
Fetal bovine serum
References
Chethan PD, Vishalakshi B, Sathish L, Ananda K, Poojary B (2013) Preparation of substituted quaternized arylfuran chitosan derivatives and their antimicrobial activity. Int J Biol Macromol 59(2013):158–164
Berger J, Reist M, Mayer JM, Felt O, Gurny R (2004) Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm 57(1):35–52
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Yamamoto H, Amaike M (1997) Biodegradation of cross-linked chitosan gels by a microorganism. Macromolecules 30(13):3936–3937
Geng X, Oh-Hyeong K, Jang J (2005) Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 26(27):5427–5432
Pasanphan W, Buettner GR, Chirachanchai S (2010) Chitosan gallate as a novel potential polysaccharide antioxidant: an EPR study. Carbohydr Res 345(1):132–140
Zargar V, Asghari M, Dashti A (2015) A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev 2(3):204–226
Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z (2008) Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev 60(15):1650–1662
Kesisoglou F, Panmai S, Wu Y (2007) Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev 59(7):631–644
Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T (2000) Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur J Pharm Biopharm 50(1):147–160
Xu Y, Du Y, Huang R, Gao L (2003) Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 24(27):5015–5022
Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W (2006) Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J. Controll Release 111(1–2):107–116
Alonso-Sande M, Cuna M, Remunan-Lopez C, Teijeiro-Osorio D, Alonso-Lebrero JL, Alonso MJ (2006) Formation of new glucomannan-chitosan nanoparticles and study of their ability to associate and deliver proteins. Macromolecules 39(12):4152–4158
Divya K, Jisha MS (2018) Chitosan nanoparticles preparation and applications. Env Chem Lett 16(1):101–112
Karathanos VT, Mourtzinos I, Yannakopoulou K, Andrikopoulos NK (2007) Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with b-cyclodextrin. Food Chem 101(2):652–658. https://doi.org/10.1016/j.foodchem.2006.01.053
Peng H, Xiong H, Li J, Xie M, Liu Y, Bai C, Chen L (2010) Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem 121(1):23–28
Li PW, Wang G, Yang ZM, Duan W, Peng Z, Kong LX, Wang QH (2016) Development of drug-loaded chitosan-vanillin nanoparticles and its cytotoxicity against HT-29 cells. Drug Deliv 23(1):30–35
Kwon J, Kim J, Park S, Khang G, Kang PM, Lee D (2013) Inflammation-responsive antioxidant nanoparticles based on a polymeric prodrug of vanillin. Biomacromolecules 14(5):1618–1626
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci Technol 28(1):25–30
Chen F, Shi Z, Neoh KG, Kang ET (2009) Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol Bioeng 104(1):30–39
Mosmann T (1983) Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Sukhramani PS, Patel PM (2013) Biological screening of Avicennia marina for anticancer activity. Der Pharm Sin 4(2):125–130
Thyriyalakshmi P, Radha KV (2015) Synthesis of dimethyl carbonate (DMC) based biodegradable nitrogen mustard ionic carbonate (NMIC) nanoparticles. RSC Adv 5:10560–10566
Aricò F, Chiurato M, Peltier J, Tundo P (2012) Sulfur and nitrogen mustard carbonate analogues. Eur J Org Chem 17:3223–3228
Jin X, Wang J, Bai J (2009) Synthesis and antimicrobial activity of the Schiff base from chitosan and citral. Carbohydr Res 344(6):825–829
Laus R, Favere VTD (2011) Competitive adsorption of Cu(II) and Cd(II) ions by chitosan crosslinked with epichlorohydrin–triphosphate. Bioresour Technol 102(19):8769–8776
Kocak N, Sahin M, Kucukkolbasi S, Erdogan ZO (2012) Synthesis and characterization of novel nano-chitosan Schiff base and use of lead (II) sensor. Int J Biol Macromol 51(5):1159–1166
Wang G, Li P, Peng Z, Huang M, Kong L (2011) Formulation of vanillin cross-linked chitosan nanoparticles and its characterization. Adv Mater Res 335–336(2011):474–477
Santos JED, Dockal ER, Cavalheir ETG (2005) Synthesis and characterization of Schiff bases from chitosan and salicylaldehyde derivatives. Carbohydr Polym 60(3):277–282
Jagadish RS, Divyashree KN, Viswanath P, Srinivas P, Raj B (2012) Preparation of N-vanillyl chitosan and 4-hydroxybenzyl chitosan and their physico-mechanical, optical, barrier, and antimicrobial properties. Carbohydr Polym 87(1):110–116
Ghate M, Manohar D, Kulkarni V, Shobhaand R, Kattimani SY (2003) Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem 38(3):297–302
Stanzione JF III, Sadler JM, La Scala JJ, Reno KH, Wool RP (2012) Vanillin-based resin for use in composite applications. Green Chem 14(8):2346–2352
Fache M, Darroman E, Besse V, Auvergne R, Caillol S, Boutevin B (2014) Vanillin, a promising biobased building-block for monomer synthesis. Green Chem 16(4):1987–1998
Krishnapriya KR, Kandaswamy M (2010) A new chitosan biopolymer derivative as metal-complexing agent: synthesis, characterization, and metal(II) ion adsorption studies. Carbohydr Res 345(14):2013–2022
Amarasekara AS, Razzaq A (2012) Vanillin-based polymers—part II: synthesis of Schiff base polymers of divanillin and their chelation with metal ions. ISRN Polym Sci 2012:1–5
Kumar S, Dutta J, Dutta PK (2009) Preparation and characterization of N-heterocyclic chitosan derivative based gels for biomedical applications. Int J Biol Macromol 45(4):330–337
Stroescu M, Stoica-Guzun A, Isopencu G, Jinga SI, Parvulescu O, Dobre T, Vasilescu M (2015) Chitosan-vanillin composites with antimicrobial properties. Food Hydrocoll 48:62–71
Marin L, Stoica I, Mares M, Dinu V, Simionescu BC, Barboiu M (2013) Antifungal vanillin–imino-chitosan biodynameric films. J Mater Chem B 1(27):3353–3358
Singh RK, Kukrety A, Chatterjee AK, Thakre GD, Bahuguna GM, Saran S, Adhikarin DK, Atray N (2014) Use of an acylated chitosan schiff base as an ecofriendly multifunctional biolubricant additive. Ind Eng Chem Res 53(48):18370–18379
Kayaci F, Uyar T (2011) Solid inclusion complexes of vanillin with cyclodextrins: their formation, characterization, and high-temperature stability. J Agric Food Chem 59(21):11772–11778
Tirkistani FAA (1998) Thermal analysis of some chitosan Schiff bases. Polym Degrad Stab 60(1):67–70
Tree-udom T, Wanichwecharungruang SP, Seemork J, Arayachukeat S (2011) Fragrant chitosan nanospheres: controlled release systems with physical and chemical barriers. Carbohydr Polym 86(4):1602–1609
Patel Rajesh M, Patel Natvar J (2011) In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res 1:52–68
Jagtap RM, Pardeshi SK (2014) Antioxidant activity screening of a series of synthesized 2-aryl thiazolidine-4-carboxylic acids. Der Pharm Lett 6(3):137–145
Zhang Y, Feng S, Wu Q, Wang K, Yi X, Wang H, Pan Y (2011) Microwave-assisted synthesis and evaluation of naphthalimides derivatives as free radical scavengers. Med Chem Res 20(6):752–759
Mohana KN, Pradeep Kumar CB (2013) Synthesis and antioxidant activity of 2-amino-5-methylthiazol derivatives containing 1,3,4-oxadiazole-2-thiol moiety. ISRN Org Chem 2013:1–8
López-Mata MA, Ruiz-Cruz S, Silva-Beltrán NP, Ornelas-Paz JDJ, Ocaño-Higuera VM, Rodríguez-Félix F, Cira-Chávez LA, Del-Toro-Sánchez CL, Shirai K (2015) Physicochemical and antioxidant properties of chitosan films incorporated with cinnamon oil. Int J Polym Sci 2015:1–8
Zai-Qun L (2007) How many free radicals can be trapped by (hydroxylphenylimino)methylphenol in the free-radical-induced peroxidation of triolein in micelles? QSAR Comb Sci 26(4):488–495
Zhang Y, Zou B, Chen Z, Pan Y, Wang H, Liang H, Yi X (2011) Synthesis and antioxidant activities of novel 4-Schiff base-7-benzyloxy-coumarin derivatives. Bioorg Med Chem Lett 21(22):6811–6815
Guo-qing Y, Wen-yue X, Wang H, Sun Y, Hua-Zhang L (2011) Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr Polym 83(4):1787–1796
Naik N, Vijay Kumar H, Harini ST (2011) Synthesis and antioxidant evaluation of novel indole-3-acetic acid analogues. Eur J Chem 2(3):337–341
Park H, Kim S, Kim S, Song Y, Seung D, Hong K, Khang G, Lee D (2010) Antioxidant and anti-inflammatory activities of hydroxybenzyl alcohol releasing biodegradable polyoxalate nanoparticles. Biomacromol 11(8):2103–2108
Kim S, Park H, Song Y, Hong D, Kim O, Jo E, Khang G, Lee D (2011) Reduction of oxidative stress by p-hydroxybenzyl alcohol-containing biodegradable polyoxalate nanoparticulate antioxidant. Biomaterials 32(11):3021–3029
Ho K, Yazan LS, Ismail N, Ismail M (2009) Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol 33(2):155–160
Chen W, Han Y, Peng X (2014) Aromatic nitrogen mustard-based prodrugs: activity, selectivity and the mechanism of DNA cross-linking. Chem Eur J 20(24):7410–7418
Gillies ER, Fréchet JMJ (2003) A new approach towards acid sensitive copolymer micelles for drug delivery. Chem Commun 14:1640–1641
Souza AJD, Topp EM (2004) Release from polymeric prodrugs: linkages and their degradation. J Pharm Sci 93(8):1962–1979
Reddy MV, Reddy GCS, Jeong YT (2015) Molybdate sulfuric acid (MSA): an efficient reusable catalyst for the synthesis of tetrahydrobenzo[4, 5]imidazo[2,1-b]quinazolin-1(2H)-ones under solvent-free conditions and evaluation for their in vitro bioassay. RSC Adv 5(15):11423–11432
Ashutosh B, Khan I, Vivek KB, Sun CK (2017) MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J Pharmacol 12:115–118
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thyriyalakshmi, P., Radha, K.V. Fabrication of chitosan-bis (4-formyl-2 methoxy phenyl carbonate) Schiff base nanoparticles and evaluation of their antioxidant and anticancer properties. Mol Biol Rep 46, 4333–4347 (2019). https://doi.org/10.1007/s11033-019-04887-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04887-4