Abstract
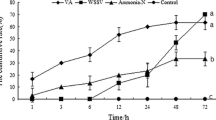
The translationally controlled tumor protein (TCTP) is a multi-functioning protein that performs vital roles, particularly in various complicated life processes. In this study, a new TCTP cDNA was cloned from Fenneropenaeus chinensis and hence was designated as Fc-TCTP. Its length is 711 bp, and it is characterized by 507-bp open reading frame that encodes a deduced 168-amino acid protein, including a TCTP domain. Moreover, this study analyzed the expression patterns of this gene when it responds to infection specifically with Vibrio anguillarum and the white spot syndrome virus (WSSV). Based on the results, Fc-TCTP was present in all the analyzed tissues. Additionally, Fc-TCTP’s expression level decreased after having been infected by bacteria, but was upregulated in the hepatopancreas after having been exposed to WSSV. Likewise, the Fc-TCTP protein was upregulated during its exposure to the virus. These results suggest that Fc-TCTP could well be involved in the antiviral response in F. chinensis.










Similar content being viewed by others
References
Chitpatima ST, Makrides S, Bandyopadhyay R, Brawerman G (1988) Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumour cells. Nucleic Acids Res 16:2350. doi:10.1093/nar/16.5.2350
Gross B, Gaestel M, Bohm H, Bielka H (1989) cDNA sequence coding for a translationally controlled human tumor protein. Nucleic Acids Res 17:8367. doi:10.1093/nar/17.20.8367
Benndorf R, Nurnberg P, Bielka H (1988) Growth phase-dependent proteins of the Ehrlich ascites tumor analyzed by one- and two-dimensional electrophoresis. Exp Cell Res 174:130–138. doi:10.1016/0014-4827(88)90148-6
Thomas G, Luther H (1981) Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci USA 78:5712–5716. doi:10.1073/pnas.78.9.5712
MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM (1995) Molecular identification of an IgE-dependent histamine-releasing factor. Science 269:688–690. doi:10.1126/science.7542803
Bommer UA, Lazaris-Karastzas A, De Benedetii A, Nurnberg P, Benndorf R, Bielka H, Sonenberg N (1994) Translational regulation of the mammalian growth-related protein p23: involvement of eLF- 4E. Cell Mol Biol Res 40:633–641
Xu A, Bellamy R, Taylor JA (1999) Expression of translationally controlled tumor protein is regulated by calcium at both the transcriptional and post-transcriptional level. Biochem J 342:683–689. doi:10.1042/0264-6021:3420683
Sturzenbaum SR, Kille P, Morgan AJ (1998) Identification of heavy metal induced changes in the expression patterns of the translationally controlled tumor protein (TCTP) in the earthworm Lumbricus rubellus. Biochim Biophys Acta 1398:294–304
Kang BS, Jang SH, Cho YJ, Yoo BJ, Yang JS (1996) Cloning and nucleotide sequence of cDNA CHK23 for translationally controlled controlled tumor protein p23 Homolog in chick embryonic myoblasts. Korean J Genet 18:143–152
Mak CH, Sun KW, Ko RC (2001) Identification of some heat-induced genes of Trichenella spiralis. Parasitology 123:293–300
Sanchez JC, Schaller D, Ravier F, Golaz O, Jaccoud S, Belet M, Wilkins MR, James R, Deshusses J, Hochstrasser D (1997) Translationally controlled tumor protein: a protein identified in several nontumoral cells including erythrocytes. Electrophoresis 18:150–155 doi:10.1002/elps.1150180127
Pay A, Heberle-Bors E, Hirt H (1992) An alfalfa cDNA encodes a protein with homology to translationally controlled human tumor protein. Plant Mol Biol 19:501–503. doi:10.1007/BF00023399
Thiele H, Berger M, Skalweit A, Thiele BJ (2000) Expression of the gene and processed pseudogenes encoding the human and rabbit translationally controlled tumour protein (TCTP). Eur J Biochem 267:5473–5481. doi:10.1046/j.1432-1327.2000.01609.x
Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P (2002) Molecular characterization of a calcium binding translationally controlled tumour protein homologue from the filarial parasites, Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol 121:107–118. doi:10.1016/S0166-6851(02)00027-0
Bommer UA, Borovjagin AV, Greagg MA, Jeffrey IW, Russell P, Laing KG, Lee M, Clemens MJ (2002) The mRNA of the translationally controlled tumour protein P23/TCTP is a highly structured RNA, which activates the dsRNA-dependent protein kinase PKR. RNA 8:478–496. doi:10.1017/S1355838202022586
Nielsen HV, Johnsen AH, Sanchez JC, Hochstrasser DF, Schiotz PO (1998) Identification of a basophil leukocyte interleukin-3-regulated protein that is identical to IgE-dependent histamine-releasing factor. Allergy 53:642–652. doi:10.1111/j.1398-9995.1998.tb03950.x
Teshima S, Rokutan K, Nikawa T, Kishi K (1998) Macrophage colony-stimulating factor stimulates synthesis and secretion of a mouse homolog of a human IgE-dependent histamine-releasing factor by macrophages in vitro and in vivo. J Immunol 161:6356–6366
Böhm H, Benndorf R, Gaestel M, Gross B, Nürnberg P, Kraft R, Otto A, Bielka H (1989) The growth-related protein P23 of the Ehrlich ascites tumor: translational control, cloning and primary structure. Biochem Int 19:277–286
Kim M, Jung Y, Lee K, Kim C (2000) Identification of the calciumb binding sites in translationally controlled tumor protein. Arch Pharm Res 23:633–636
Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer U (1999) The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci 112:1257–1271
MacDonald SM, Bhisutthibhan J, Shapiro TA, Rogerson SJ, Taylor TE, Tembo M, Langdon JM, Meshnick SR (2001) Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homologue in vitro and in vivo. Proc Natl Acad Sci USA 98:10829–10832. doi:10.1073/pnas.201191498
Schroeder JT, Lichtenstein LM, MacDonald SM (1997) Recombinant histamine-releasing factor enhances IgE-dependent IL-4 and IL-13 secretion by human basophils. J Immunol 159:447–452
Bheekha-Escura R, MacGlashan DW, Langdon JM, MacDonald SM (2000) Human recombinant histamine-releasing factor activates human eosinophils and the eosinophilic cell line, AML14-3D10. Blood 96:2191–2198
Schroeder JT, Lichtenstein LM, MacDonald SM (1996) An immunoglobulin E-dependent recombinant histamine-releasing factor induces interleukin-4 secretion from human basophils. J Exp Med 183:1265–1270. doi:10.1084/jem.183.3.1265
Kang HS, Lee MJ, Song H, Han SH, Kim YM, Im JY, Choi I (2001) Molecular identification of IgE-dependent histamine-releasing factor as a B cell growth factor. J Immunol 166:6545–6554
Cans C, Passer BJ, Shalak V, Portebois VN, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, Fiucci G, Goud B, Mirande M, Amson R, Telerman A (2003) Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation facto eEF1A. Proc Natl Acad Sci USA 100:13892–13897. doi:10.1073/pnas.2335950100
Christodoulopoulos P, Cameron L, Durham S, Hamid Q (2000) Molecular pathology of allergic disease II: Upper airway disease. J Allergy Clin Immunol 105:211–223. doi:10.1016/S0091-6749(00)90068-X
Enwonwu CO, Afolabi BM, Salako LO, Idigbe EO, Bashirelah N (2000) Increased plasma levels of histidine and histamine in flaciparum malaria: relevance to severity of infection. J Neural Transm 107:1273–1287. doi:10.1007/s007020070017
Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF (2005) Stabilization and enhancement of the antiapoptotic activity of mcl-1 by TCTP. Mol Cell Biol 25:3117–3126. doi:10.1128/MCB.25.8.3117-3126.2005
Tuynder M, Fiucci G, Prieur S, Lespagnol A, Geant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J, Moras D, Amson R, Telerman A (2004) Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci USA 101:15364–15369. doi:10.1073/pnas.0406776101
Rojtinnakorn J, Hirono I, Itami T, Takahashi Y, Aoki T (2002) Gene expression in haemocytes of kuruma prawn, Penaeus japonicus, in response to infection with WSSV by EST approach. Fish Shellfish Immunol 13:69–83. doi:10.1006/fsim.2001.0382
He N, Qin Q, Xu X (2005) Differential profile of genes expressed in hemocytes of white spot syndrome Virus-resistant shrimp (Penaeus japonicus) by combining suppression subtractive hybridization and differential hybridization. Antiviral Res 66:39–45. doi:10.1016/j.antiviral.2004.12.010
Bangrak P, Graidist P, Chotigeat W, Phongdara A (2004) Molecular cloning and expression of a mammalian homologue of a translationally controlled tumor protein (TCTP) gene from Penaeus monodon shrimp. J Biotechnol 108:219–226. doi:10.1016/j.jbiotec.2003.12.007
Graidist P, Fujise K, Wanna W, Sritunyalucksana K, Phongdara A (2006) Establishing a role for shrimp fortilin in preventing cell death. Aquaculture 255:157–164. doi:10.1016/j.aquaculture.2005.12.023
Wang YG, Hassan MD, Shariff M, Zamri SM, Chen X (1999) Histopathology and cytopathology of white spot syndrome virus (WSSV) in cultured Penaeus monodon from peninsular Malaysia with emphasis on pathogenesis and the mechanism of white spot formation. Dis Aquat Organ 39:1–11. doi:10.3354/dao039001
Dhar AK, Roux MM, Klimpel KR (2001) Detection and quantification of infectious hypodermal and hematopoietic necrosis virus and white spot virus in shrimp using real-time quantitative PCR and SYBR Green chemistry. J Clin Microbiol 39(8):2835–2845. doi:10.1128/JCM.39.8.2835-2845.2001
Zhao XF, Wang JX, Xu XL, Schmid R, Wieczorek H (2002) Molecular cloning and characterization of the cathepsin B-like proteinase from the cotton boll worm, Helicoverpa armigera. Insect Mol Biol 11:567–575. doi:10.1046/j.1365-2583.2002.00366.x
Du XJ, Zhao XF, Wang JX (2007) Molecular cloning and characterization of a lipopolysaccharide and beta-1, 3-glucan binding protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 44:1085–1094. doi:10.1016/j.molimm.2006.07.288
Sun YD, Fu LD, Jia YP, Du XJ, Wang Q, Wang YH, Zhao XF, Yu XQ, Wang JX (2008) A hepatopancreas specific C-type lectin from the Chinese shrimp Fenneropenaeus chinensis exhibits antimicrobial activity. Mol Immunol 45:348–361. doi:10.1016/j.molimm.2007.06.355
Thaw P, Baxter NJ, Hounslow AM, Price C, Waltho JP, Craven CJ (2001) Structure of TCTP reveals unexpected relationship with guanine nucleotidefree chaperones. Nat Struct Biol 8:701–704. doi:10.1038/90415
Roux MM, Pain A, Klimpel KR, Dhar AK (2002) The lipopolysaccharide and β-1, 3-glucan binding protein gene is upregulated in white spot virus-infected shrimp (Penaeus stylirostris). J Virol 76:7140–7149. doi:10.1128/JVI.76.14.7140-7149.2002
Robalino J, Bartlett TC, Chapman RW, Gross PS, Browdy CL, Warr GW (2007) Double-stranded RNA and antiviral immunity in marine shrimp: inducible host mechanisms and evidence for the evolution of viral counter-responses. Dev Comp Immunol 31:539–547. doi:10.1016/j.dci.2006.08.011
Robalino J, Browdy CL, Prior S, Metz A, Parnell P, Gross P, Warr G (2004) Induction of antiviral immunity by double-stranded RNA in a marine invertebrate. J Virol 78:10442–10448. doi:10.1128/JVI.78.19.10442-10448.2004
Schulenburg H, Boehnisch C, Michiels NK (2007) How do invertebrates generate a highly specific innate immune response? Mol Immunol 44:3338–3344. doi:10.1016/j.molimm.2007.02.019
Grebenjuk VA, Kuusksalu A, Kelve M, Schutze J, Schroder HC, Muller WE (2002) Induction of (2–5)oligoadenylate synthetase in the marine sponges Suberites domuncula and Geodia cydonium by the bacterial endotoxin lipopolysaccharide. Eur J Biochem 269:1382–1392. doi:10.1046/j.1432-1033.2002.02781.x
Agaisse H, Perrimon N (2004) The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev 198:72–82. doi:10.1111/j.0105-2896.2004.0133.x
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30770282) and the National High Technology Research and Development Program of China (863 Program) (No. 2007AA09Z425) and and the Ph.D. program foundation of the Ministry of Education of China (No. 20060422034).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Zhao, XF. & Wang, JX. Molecular cloning and characterization of the translationally controlled tumor protein from Fenneropenaeus chinensis . Mol Biol Rep 36, 1683–1693 (2009). https://doi.org/10.1007/s11033-008-9369-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9369-2