Abstract
N-furfuryl piperazine ureas disclosed by scientists at GSK Tres Cantos were chosen as antimycobacterial hits from a phenotypic whole-cell screen. Bioisosteric replacement of the furan ring in the GSK Tres Cantos molecules with a phenyl ring led to molecule (I) with an MIC of 1 μM against Mtb H37Rv, low cellular toxicity (HepG2 IC50 ~ 80 μM), good DMPK properties and specificity for Mtb. With the aim of delineating the SAR associated with (I), fifty-five analogs were synthesized and screened against Mtb. The SAR suggests that the piperazine ring, benzyl urea and piperonyl moieties are essential signatures of this series. Active compounds in this series are metabolically stable, have low cellular toxicity and are valuable leads for optimization. Molecular docking suggests these molecules occupy the Q0 site of QcrB like Q203.
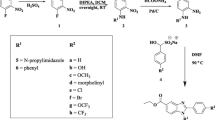
Graphic Abstract
Bioisosteric replacement of N-furfuryl piperazine-1-carboxamides yielded molecule (I) a novel lead with satisfactory PD, metabolism, and toxicity profiles.








Similar content being viewed by others
References
WHO Global Tuberculosis Report 2018 (2018). https://www.who.int/tb/publications/global_report/en/
Rebollo-Lopez MJ, Lelievre J, Alvarez-Gomez D, Castro-Pichel J, Martinez-Jimenez F, Papadatos G, Kumar V, Colmenarejo G, Mugumbate G, Hurle M (2015) Release of 50 new, drug-like compounds and their computational target predictions for open source anti-tubercular drug discovery. PLoS ONE 10(12):e0142293
Ballell L, Bates RH, Young RJ, Alvarez-Gomez D, Alvarez-Ruiz E, Barroso V, Blanco D, Crespo B, Escribano J, González R (2013) Fueling open-source drug discovery: 177 small-molecule leads against tuberculosis. ChemMedChem 8(2):313–321
Arora K, Ochoa-Montaño B, Tsang PS, Blundell TL, Dawes SS, Mizrahi V, Bayliss T, Mackenzie CJ, Cleghorn LA, Ray PC (2014) Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58(11):6962–6965
Foo CS, Lupien A, Kienle M, Vocat A, Benjak A, Sommer R, Lamprecht DA, Steyn AJ, Pethe K, Piton J (2018) Arylvinylpiperazine amides, a new class of potent inhibitors targeting QcrB of mycobacterium tuberculosis. MBio 9(5):e01276-01218
Peterson LA (2012) Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem Res Toxicol 26(1):6–25
Schultz TW, Yarbrough JW, Hunter RS, Aptula AO (2007) Verification of the structural alerts for Michael acceptors. Chem Res Toxicol 20(9):1359–1363
Ollinger J, Bailey MA, Moraski GC, Casey A, Florio S, Alling T, Miller MJ, Parish T (2013) A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS ONE 8(4):e60531
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes I. Evidence for its hemoprotein nature. J Biol Chem 239(7):2370–2378
Ko Y, Choi I (2016) Putative 3D structure of QcrB from mycobacterium tuberculosis cytochrome bc 1 complex, a novel drug-target for new series of antituberculosis agent Q203. Bull Korean Chem Soc 37(5):725–731
Kovacs Z, Sherry AD (1997) pH-controlled selective protection of polyaza macrocycles. Synthesis 07:759–763
Gruber B, Balk S, Stadlbauer S, König B (2012) Dynamic interface imprinting: high-affinity peptide binding sites assembled by analyte-induced recruiting of membrane receptors. Angew Chem Int Ed 51(40):10060–10063
Broggini G, Barbera V, Beccalli EM, Chiacchio U, Fasana A, Galli S, Gazzola S (2013) Selective intramolecular palladium (II)-catalyzed aminooxygenation vs. diamination of alkenylureas: efficient microwave-assisted reactions to bicyclic piperazinones. Adv Synth Catal 355(8):1640–1648
Giofre S, Beccalli EM, Foschi F, La Rosa C, Presti LL, Christodoulou MS (2019) Chemo-and regioselective palladium (II)-catalyzed aminoarylation of N-allylureas providing 4-arylmethyl imidazolidinones. Synthesis 51(18):3462–3470
Borsini E, Broggini G, Fasana A, Galli S, Khansaa M, Piarulli U, Rigamonti M (2011) Intramolecular palladium-catalyzed aminocarboxylation of olefins as a direct route to bicyclic oxazolidinones. Adv Synth Catal 353(6):985–994
Jang HY, Park HJ, Damodar K, Kim J-K, Jun J-G (2016) Dihydrostilbenes and diarylpropanes: synthesis and in vitro pharmacological evaluation as potent nitric oxide production inhibition agents. Bioorg Med Chem Lett 26(22):5438–5443
Wu D, Zhang T, Chen Y, Huang Y, Geng H, Yu Y, Zhang C, Lai Z, Wu Y, Guo X (2017) Discovery and optimization of chromeno[2, 3-c]pyrrol-9 (2 H)-ones as novel selective and orally bioavailable phosphodiesterase 5 inhibitors for the treatment of pulmonary arterial hypertension. J Med Chem 60(15):6622–6637
Corsano S, Strappaghetti G, Ferrini R, Sala R (1988) Synthesis of compounds with a possible Ca-antagonist and β-blocking activity. Arch Pharm 321(10):731–734
Price KE, Larrivee-Aboussafy C, Lillie BM, McLaughlin RW, Mustakis J, Hettenbach KW, Hawkins JM, Vaidyanathan R (2009) Mild and efficient DBU-catalyzed amidation of cyanoacetates. Org Lett 11(9):2003–2006
Eswar N, Webb B, Marti-Renom MA, Madhusudhan M, Eramian D, My S, Pieper U, Sali A (2006) Comparative protein structure modeling using modeller. Curr Prot Bioinform 15(1):5.6.1–5.6.30
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Peram MR, Jalalpure S, Kumbar V, Patil S, Joshi S, Bhat K, Diwan P (2019) Factorial design based curcumin ethosomal nanocarriers for the skin cancer delivery: in vitro evaluation. J Liposome Res 29(3):291–311
Walawalkar PS, Serai PS, Iyer K (2006) Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci 68(2):262–265
Acknowledgements
We thankfully acknowledge the National Facility for Biopharmaceuticals, G N Khalsa College, Mumbai, for antibacterial activity results; Maratha Mandal’s Central Research Laboratory, Belgaum, for cytotoxicity results; SAIF, Panjab University, Chandigarh, for HRMS analysis, Mrs. Varsha Vhadge, Ambernath Organics Pvt. Ltd, Mumbai, for HPLC purity results and Dr. M. K Rangnekar Memorial testing laboratory, Bombay College of Pharmacy, Mumbai, for FTIR analysis.
Funding
Financial support for this work was provided by Ambernath Organics Pvt. Ltd. and the Amrut Mody Research Foundation at Bombay College of Pharmacy.
Author information
Authors and Affiliations
Contributions
SS and AAK wrote the paper and synthesized the molecules. RC, AK, BS synthesized the molecules. JP oversaw the synthesis and analysis. DD, KR, KRI performed metabolism studies. EAFM performed computational studies. AK and TP antimycobacterial assay (H37Rv strain). ECC and SN designed and oversaw the study.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satish, S., Chitral, R., Kori, A. et al. Design, synthesis and SAR of antitubercular benzylpiperazine ureas. Mol Divers 26, 73–96 (2022). https://doi.org/10.1007/s11030-020-10158-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10158-3