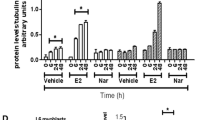
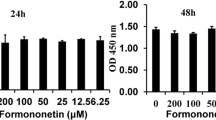
Abstract
Skeletal muscle regeneration is a complex process, involving the proliferation, migration, and differentiation of myoblasts. Recent studies suggest that some natural flavanones stimulate myogenesis. However, the effect of plant estrogen, silibinin, on the regulation of myoblast behaviors is unclarified. In this study, we investigated the effects of silibinin on immortalized murine myoblast C2C12 in the aspects of proliferation, migration, differentiation along with underlying mechanisms. The results show that silibinin at concentrations below 50 μM enhanced the migration and differentiation of C2C12 cells, but had no effect on cell proliferation. Silibinin significantly promoted the production of ROS, which appeared to play important roles in the migration and differentiation of the myoblasts. Interestingly, among ROS, the superoxide anion and hydroxyl radical were associated with the migration, whereas hydrogen peroxide contributed to the myogenic differentiation. We used ER agonist and antagonist to explore whether estrogen receptors (ERs), which are affected by silibinin treatment in the silibinin-enhanced C2C12 migration and differentiation. Migration was independent of ERs, whereas the differentiation was associated with decreased ERα activity. In summary, silibinin treatment increases ROS levels, leading to the promotion of migration and myogenic differentiation. Negative regulation ERα of differentiation but not of migration may suggest that ERα represses hydrogen peroxide generation. The effect of silibinin on myoblast migration and differentiation suggests that silibinin may have therapeutic benefits for muscle regeneration.











Similar content being viewed by others
References
Prior B, Modlesky C, Evans E, Sloniger M, Saunder M, Lewis R, Cureton K (2001) Muscularity and the density of the fat-free mass in athletes. J Appl Physiol 90:1523–1531. https://doi.org/10.1152/jappl.2001.90.4.1523
Baghdadi MB, Tajbakhsh S (2018) Regulation and phylogeny of skeletal muscle regeneration. Dev Biol 433:200–209. https://doi.org/10.1016/j.ydbio.2017.07.026
Braun T, Gautel M (2011) Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12:349–361. https://doi.org/10.1038/nrm3118
Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M (2009) 17Beta-estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha-mediated signals. Am J Physiol Cell Physiol 297:C1249–C1262. https://doi.org/10.1152/ajpcell.00188.2009
Ogawa M, Yamaji R, Higashimura Y, Harada N, Ashida H, Nakano Y, Inui H (2011) 17Beta-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor alpha. J Biol Chem 286:41455–41465. https://doi.org/10.1074/jbc.M111.276824
Zhou DR, Eid R, Miller KA, Boucher E, Mandato CA, Greenwood MT (2019) Intracellular second messengers mediate stress inducible hormesis and Programmed Cell Death: a review. Biochim Biophys Acta Mol Cell Res 1866:773–792. https://doi.org/10.1016/j.bbamcr.2019.01.016
Urao N, Ushio-Fukai M (2013) Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med 54:26–39. https://doi.org/10.1016/j.freeradbiomed.2012.10.532
Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS (2011) Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci USA 108:16098–16103. https://doi.org/10.1073/pnas.1109546108
Youm TH, Woo SH, Kwon ES, Park SS (2019) NADPH oxidase 4 contributes to myoblast fusion and skeletal muscle regeneration. Oxid Med Cell Longev 2019:3585390. https://doi.org/10.1155/2019/3585390
Le Moal E, Pialoux V, Juban G, Groussard C, Zouhal H, Chazaud B, Mounier R (2017) Redox control of skeletal muscle regeneration. Antioxid Redox Signal 27:276–310. https://doi.org/10.1089/ars.2016.6782
Lee S, Tak E, Lee J, Rashid MA, Murphy MP, Ha J, Kim SS (2011) Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res 21:817–834. https://doi.org/10.1038/cr.2011.55
Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K, Seino S, Takahashi Y (2011) Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 152:912–921. https://doi.org/10.1210/en.2010-0981
Bosutti A, Degens H (2015) The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep 5:80–93. https://doi.org/10.1038/srep08093
Powers SK, Talbert EE, Adhihetty PJ (2011) Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589:2129–2138. https://doi.org/10.1113/jphysiol.2010.201327
Lim S, Shin JY, Jo A, Jyothi KR, Nguyen MN, Choi TG, Kim J, Park JH, Eun YG, Yoon KS, Ha J, Kim SS (2013) Carbonyl reductase 1 is an essential regulator of skeletal muscle differentiation and regeneration. Int J Biochem Cell Biol 45:1784–1793. https://doi.org/10.1016/j.biocel.2013.05.025
Hansen JM, Klass M, Harris C, Csete M (2007) A reducing redox environment promotes C2C12 myogenesis: implications for regeneration in aged muscle. Cell Biol Int 31:546–553. https://doi.org/10.1016/j.cellbi.2006.11.027
Ferreira LF, Reid MB (1985) (2008) Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol 104:853–860. https://doi.org/10.1152/japplphysiol.00953.2007
Sun Y, Yang J, Liu W, Yao G, Xu F, Hayashi T, Onodera S, Ikejima T (2019) Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic beta-cells is mediated by estrogen receptor alpha. Mol Cell Biochem 460:81–92. https://doi.org/10.1007/s11010-019-03572-1
Zheng N, Liu L, Liu WW, Li F, Hayashi T, Tashiro SI, Onodera S, Ikejima T (2017) Crosstalk of ROS/RNS and autophagy in silibinin-induced apoptosis of MCF-7 human breast cancer cells in vitro. Acta Pharmacol Sin 38:277–289. https://doi.org/10.1038/aps.2016.117
Acharya S, Stark TD, Oh ST, Jeon S, Pak SC, Kim M, Hur J, Matsutomo T, Hofmann T, Hill RA, Balemba OB (2017) (2R,3S,2″'R,3″R)-manniflavanone protects proliferating skeletal muscle cells against oxidative stress and stimulates myotube formation. J Agric Food Chem 65:3636–3646. https://doi.org/10.1021/acs.jafc.6b05161
Nie Y, Chen H, Guo C, Yuan Z, Zhou X, Zhang Y, Zhang X, Mo D, Chen Y (2017) Palmdelphin promotes myoblast differentiation and muscle regeneration. Sci Rep 7:41608. https://doi.org/10.1038/srep41608
Neuhaus P, Oustanina S, Loch T, Kruger M, Bober E, Dono R, Zeller R, Braun T (2003) Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol Cell Biol 23:6037–6048. https://doi.org/10.1128/mcb.23.17.6037-6048.2003
Dedieu S, Poussard S, Mazeres G, Grise F, Dargelos E, Cottin P, Brustis JJ (2004) Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp Cell Res 292:187–200. https://doi.org/10.1016/j.yexcr.2003.08.014
Gao Z, Liu R, Ye N, Liu C, Li X, Guo X, Zhang Z, Li X, Yao Y, Jiang X (2018) FOXO1 inhibits tumor cell migration via regulating cell surface morphology in non-small cell lung cancer cells. Cell Physiol Biochem 48:138–148. https://doi.org/10.1159/000491670
Malewicz B, Wang Z, Jiang C, Guo J, Cleary MP, Grande JP, Lu J (2006) Enhancement of mammary carcinogenesis in two rodent models by silymarin dietary supplements. Carcinogenesis 27:1739–1747. https://doi.org/10.1093/carcin/bgl032
Dupuis ML, Conti F, Maselli A, Pagano MT, Ruggieri A, Anticoli S, Fragale A, Gabriele L, Gagliardi MC, Sanchez M, Ceccarelli F, Alessandri C, Valesini G, Ortona E, Pierdominici M (2018) The natural agonist of estrogen receptor beta silibinin plays an immunosuppressive role representing a potential therapeutic tool in rheumatoid arthritis. Front Immunol 9:1903. https://doi.org/10.3389/fimmu.2018.01903
Bosutti A, Degens H (2015) The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep 5:8093. https://doi.org/10.1038/srep08093
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44:532–553. https://doi.org/10.1159/000485089
Liu C, McFarland D, Velleman S (2005) Effect of genetic selection on MyoD and myogenin expression in turkeys with different growth rates. Poult Sci 84:376–384. https://doi.org/10.1093/ps/84.3.376
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859. https://doi.org/10.1182/blood-2004-09-3662
Malinska D, Kudin AP, Bejtka M, Kunz WS (2012) Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion 12:144–148. https://doi.org/10.1016/j.mito.2011.06.015
Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME (2006) The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17:3978–3988. https://doi.org/10.1091/mbc.e05-06-0532
Ogawa M, Kitano T, Kawata N, Sugihira T, Kitakaze T, Harada N, Yamaji R (2017) Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor beta and increases skeletal muscle mass in young female mice. J Nutr Biochem 49:63–70. https://doi.org/10.1016/j.jnutbio.2017.07.017
Fan S, Qi M, Yu Y, Li L, Yao G, Tashiro S, Onodera S, Ikejima T (2012) P53 activation plays a crucial role in silibinin induced ROS generation via PUMA and JNK. Free Radic Res 46:310–319. https://doi.org/10.3109/10715762.2012.655244
Goncalves RLS, Watson MA, Wong HS, Orr AL, Brand MD (2020) The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol 28:101341. https://doi.org/10.1016/j.redox.2019.101341
Forman H, Fukuto J, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:246–256. https://doi.org/10.1152/ajpcell.00516.2003
Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C (2002) Production of interleukin-6 by skeletal myotubes role of reactive oxygen species. Am J Respir Cell Mol Biol 26:587–593. https://doi.org/10.1165/ajrcmb.26.5.4598
Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, LLeonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12:376–390. https://doi.org/10.1016/j.arr.2012.10.004
Touyz R (2005) Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 7:1302–1313. https://doi.org/10.1089/ars.2005.7.1302
Vara D, Pula G (2014) Reactive oxygen species: physiological roles in the regulation of vascular cells. Curr Mol Med 14:1103–1125. https://doi.org/10.2174/1566524014666140603114010
Tamborindeguy MT, Matte BF, Ramos GO, Alves AM, Bernardi L, Lamers ML (2018) NADPH-oxidase-derived ROS alters cell migration by modulating adhesions dynamics. Biol Cell 110:225–236. https://doi.org/10.1111/boc.201800011
L’Honore A, Drouin J, Buckingham M, Montarras D (2014) Pitx2 and Pitx3 transcription factors: two key regulators of the redox state in adult skeletal muscle stem cells and muscle regeneration. Free Radic Biol Med 75(Suppl 1):S21–S53. https://doi.org/10.1016/j.freeradbiomed.2014.10.781
L’Honore A, Commere PH, Negroni E, Pallafacchina G, Friguet B, Drouin J, Buckingham M, Montarras D (2018) The role of Pitx2 and Pitx3 in muscle stem cells gives new insights into P38alpha MAP kinase and redox regulation of muscle regeneration. eLife 7. https://doi.org/10.7554/eLife.32991
Aoyama S, Jia H, Nakazawa K, Yamamura J, Saito K, Kato H (2016) Dietary genistein prevents denervation-induced muscle atrophy in male rodents via effects on estrogen receptor-alpha. J Nutr 146:1147–1154. https://doi.org/10.3945/jn.115.226316
Vasconsuelo A, Milanesi L, Boland R (2010) Participation of HSP27 in the antiapoptotic action of 17beta-estradiol in skeletal muscle cells. Cell Stress Chaperones 15:183–192. https://doi.org/10.1007/s12192-009-0132-y
Li QY, Chen L, Zhu YH, Zhang M, Wang YP, Wang MW (2011) Involvement of estrogen receptor-beta in farrerol inhibition of rat thoracic aorta vascular smooth muscle cell proliferation. Acta Pharmacol Sin 32:433–440. https://doi.org/10.1038/aps.2011.1
Hsieh DJ, Kuo WW, Lai YP, Shibu MA, Shen CY, Pai P, Yeh YL, Lin JY, Viswanadha VP, Huang CY (2015) 17Beta-estradiol and/or estrogen receptor beta attenuate the autophagic and apoptotic effects induced by prolonged hypoxia through HIF-1alpha-mediated BNIP3 and IGFBP-3 signaling blockage. Cell Physiol Biochem 36:274–284. https://doi.org/10.1159/000374070
Ambhore NS, Katragadda R, Raju Kalidhindi RS, Thompson MA, Pabelick CM, Prakash YS, Sathish V (2018) Estrogen receptor beta signaling inhibits PDGF induced human airway smooth muscle proliferation. Mol Cell Endocrinol 476:37–47. https://doi.org/10.1016/j.mce.2018.04.007
Fan D, Liu SY, van Hasselt CA, Vlantis AC, Ng EK, Zhang H, Dong Y, Ng SK, Chu R, Chan AB, Du J, Wei W, Liu X, Liu Z, Xing M, Chen GG (2015) Estrogen receptor alpha induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab 100:E561–E571. https://doi.org/10.1210/jc.2014-3257
Nadal-Serrano M, Sastre-Serra J, Pons DG, Miro AM, Oliver J, Roca P (2012) The ERalpha/ERbeta ratio determines oxidative stress in breast cancer cell lines in response to 17beta-estradiol. J Cell Biochem 113:3178–3185. https://doi.org/10.1002/jcb.24192
Cook KL, Clarke PA, Parmar J, Hu R, Schwartz-Roberts JL, Abu-Asab M, Warri A, Baumann WT, Clarke R (2014) Knockdown of estrogen receptor-alpha induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. FASEB J 28:3891–3905. https://doi.org/10.1096/fj.13-247353
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Long, X., Gao, Y., Liu, W. et al. Natural flavonoid silibinin promotes the migration and myogenic differentiation of murine C2C12 myoblasts via modulation of ROS generation and down-regulation of estrogen receptor α expression. Mol Cell Biochem 474, 243–261 (2020). https://doi.org/10.1007/s11010-020-03849-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03849-w