Abstract
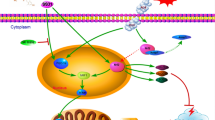
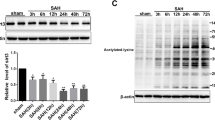
Early brain injury (EBI) was reported to be the primary cause of high mortality and poor outcomes in subarachnoid hemorrhage (SAH) patients, and apoptosis is regarded as the most important physiopathologic mechanism during EBI. Recently, our team found that thioredoxin-interacting protein (TXNIP) links endoplasmic reticulum stress (ER stress) to neuronal apoptosis and aggravates EBI. However, the other underlying mechanisms remain unknown. Mitochondria are considered to be the central points in integrating apoptotic cell death. However, whether crosstalk between TXNIP and the mitochondria-mediated intrinsic apoptotic pathway is effective on EBI has not been previously reported. Therefore, we created an endovascular perforation SAH model in Sprague–Dawley rats to determine the possible mechanism. We found that TXNIP expression in apoptotic neurons significantly increased in the SAH group compared with the sham group. In addition, increased TXNIP expression was accompanied by remarkable changes in mitochondrial-related antiapoptotic and proapoptotic factors. Furthermore, resveratrol (RES, a TXNIP inhibitor) administration significantly downregulated the expression of TXNIP and mitochondria-related proapoptotic factors. Additionally, it attenuated SAH prognostic indicators, such as brain edema, blood–brain barrier permeability, and neurological deficits. Therefore, our study further confirms that TXNIP may participate in neuronal apoptosis through the mitochondrial signaling pathway and that TXNIP may be a target for SAH treatment.





Similar content being viewed by others
References
Haut SR, Bigal ME, Lipton RB (2006) Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol 5:148–157. https://doi.org/10.1016/s1474-4422(06)70348-9
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4:432–446. https://doi.org/10.1007/s12975-013-0257-2
He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH (2012) CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke 43:484–490. https://doi.org/10.1161/strokeaha.111.626432
Guo Z, Hu Q, Xu L, Guo ZN, Ou Y, He Y, Yin C, Sun X, Tang J, Zhang JH (2016) Lipoxin A4 reduces inflammation through formyl peptide receptor 2/p38 MAPK signaling pathway in subarachnoid hemorrhage rats. Stroke 47:490–497. https://doi.org/10.1161/strokeaha.115.011223
Sehba FA, Pluta RM, Zhang JH (2011) Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol 43:27–40. https://doi.org/10.1007/s12035-010-8155-z
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97:14–37. https://doi.org/10.1016/j.pneurobio.2012.02.003
Benfica PL, Avila RI, Rodrigues BDS, Cortez AP, Batista AC, Gaeti MPN, Lima EM, Rezende KR, Valadares MC (2017) 4-Nerolidylcatechol: apoptosis by mitochondrial mechanisms with reduction in cyclin D1 at G0/G1 stage of the chronic myelogenous K562 cell line. Pharm Biol 55:1899–1908. https://doi.org/10.1080/13880209.2017.1311351
Nabatchian F, Moradi A, Aghaei M, Ghanadian M, Jafari SM, Tabesh S (2017) New 6(17)-epoxylathyrane diterpene: aellinane from Euphorbia aellenii induces apoptosis via mitochondrial pathway in ovarian cancer cell line. Toxicol Mech Methods 27:622–630. https://doi.org/10.1080/15376516.2017.1347735
Yu K, Wang T, Li Y, Wang C, Wang X, Zhang M, Xie Y, Li S, An Z, Ye T (2017) Niclosamide induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in human thyroid cancer in vitro. Biomed Pharmacother 92:403–411. https://doi.org/10.1016/j.biopha.2017.05.097
Huang L, Zhang T, Li S, Duan J, Ye F, Li H, She Z, Gao G, Yang X (2014) Anthraquinone G503 induces apoptosis in gastric cancer cells through the mitochondrial pathway. PLoS ONE 9:e108286. https://doi.org/10.1371/journal.pone.0108286
Shalev A (2014) Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol Endocrinol 28:1211–1220. https://doi.org/10.1210/me.2014-1095
Xavier JM, Rodrigues CM, Sola S (2016) Mitochondria: major regulators of neural development. Neuroscientist 22:346–358. https://doi.org/10.1177/1073858415585472
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. https://doi.org/10.1038/nature05292
Ye X, Zuo D, Yu L, Zhang L, Tang J, Cui C, Bao L, Zan K, Zhang Z, Yang X, Chen H, Tang H, Zu J, Shi H, Cui G (2017) ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem Biophys Res Commun 485:499–505. https://doi.org/10.1016/j.bbrc.2017.02.019
Li J, Yue Z, Xiong W, Sun P, You K, Wang J (2017) TXNIP overexpression suppresses proliferation and induces apoptosis in SMMC7221 cells through ROS generation and MAPK pathway activation. Oncol Rep 37:3369–3376. https://doi.org/10.3892/or.2017.5577
Wei M, Jiao D, Han D, Wu J, Wei F, Zheng G, Guo Z, Xi W, Yang F, Xie P, Zhang L, Yang AG, Wang H, Qin W, Wen W (2017) Knockdown of RNF2 induces cell cycle arrest and apoptosis in prostate cancer cells through the upregulation of TXNIP. Oncotarget 8:5323–5338. https://doi.org/10.18632/oncotarget.14142
Ishrat T, Mohamed IN, Pillai B, Soliman S, Fouda AY, Ergul A, El-Remessy AB, Fagan SC (2015) Thioredoxin-interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol Neurobiol 51:766–778. https://doi.org/10.1007/s12035-014-8766-x
Saxena G, Chen J, Shalev A (2010) Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem 285:3997–4005. https://doi.org/10.1074/jbc.M109.034421
Zhao Q, Che X, Zhang H, Fan P, Tan G, Liu L, Jiang D, Zhao J, Xiang X, Liang Y, Sun X, He Z (2017) Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J Neuroinflamm 14:104. https://doi.org/10.1186/s12974-017-0878-6
Zhao Q, Che X, Zhang H, Tan G, Liu L, Jiang D, Zhao J, Xiang X, Sun X, He Z (2017) Thioredoxin-interacting protein mediates apoptosis in early brain injury after subarachnoid haemorrhage. Int J Mol Sci. https://doi.org/10.3390/ijms18040854
Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Yegen BC, Sener G (2009) Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J Pineal Res 46:324–332. https://doi.org/10.1111/j.1600-079X.2009.00664.x
Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH (2011) alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 42:3530–3536. https://doi.org/10.1161/strokeaha.111.619965
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167:327–334. https://doi.org/10.1016/j.jneumeth.2007.08.004
Lanzillotta A, Pignataro G, Branca C, Cuomo O, Sarnico I, Benarese M, Annunziato L, Spano P, Pizzi M (2013) Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol Dis 49:177–189. https://doi.org/10.1016/j.nbd.2012.08.018
Shin JA, Lee H, Lim YK, Koh Y, Choi JH, Park EM (2010) Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J Neuroimmunol 227:93–100. https://doi.org/10.1016/j.jneuroim.2010.06.017
Xi G, Hua Y, Keep RF, Younger JG, Hoff JT (2002) Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir Suppl 81:253–256
Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH (2006) Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke 37:1888–1894. https://doi.org/10.1161/01.str.0000227259.15506.24
Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH (2011) Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke 42:484–490. https://doi.org/10.1161/strokeaha.110.604421
Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH (2007) The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res 1136:200–207. https://doi.org/10.1016/j.brainres.2006.12.023
Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH (2010) Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med 38:572–578. https://doi.org/10.1097/CCM.0b013e3181cb1158
Nakatsuka H, Ohta S, Tanaka J, Toku K, Kumon Y, Maeda N, Sakanaka M, Sakaki S (1999) Release of cytochrome c from mitochondria to cytosol in gerbil hippocampal CA1 neurons after transient forebrain ischemia. Brain Res 849:216–219
Yan J, Liu XH, Han MZ, Wang YM, Sun XL, Yu N, Li T, Su B, Chen ZY (2015) Blockage of GSK3beta-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer’s disease. Neurobiol Aging 36:211 – 27 doi. https://doi.org/10.1016/j.neurobiolaging.2014.08.005
Hu Y, Li C, Li H, Li M, Shu X (2014) Resveratrol-mediated reversal of tumor multi-drug resistance. Curr Drug Metab 15:703–710
Nivet-Antoine V, Cottart CH, Lemarechal H, Vamy M, Margaill I, Beaudeux JL, Bonnefont-Rousselot D, Borderie D (2010) trans-Resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie 92:1766–1771. https://doi.org/10.1016/j.biochi.2010.07.018
Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R (2003) Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. Faseb J 17:2339–2341. https://doi.org/10.1096/fj.03-0292fje
Bedarida T, Baron S, Vibert F, Ayer A, Henrion D, Thioulouse E, Marchiol C, Beaudeux JL, Cottart CH, Nivet-Antoine V (2016) Resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J Gerontol A 71:720–729. https://doi.org/10.1093/gerona/glv071
Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ (2014) Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci 52:286–293. https://doi.org/10.1007/s12031-013-0141-2
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28:399–414. https://doi.org/10.1179/016164106x115008
Wang X, Wang M, Xu J, Jia Z, Liu Z, Wang L, Song L (2017) Soluble adenylyl cyclase mediates mitochondrial pathway of apoptosis and ATP metabolism in oyster Crassostrea gigas exposed to elevated CO2. Fish Shellfish Immunol 66:140–147. https://doi.org/10.1016/j.fsi.2017.05.002
Simula L, Nazio F, Campello S (2017) The mitochondrial dynamics in cancer and immune-surveillance. Semin Cancer Biol 47:29–42. https://doi.org/10.1016/j.semcancer.2017.06.007
Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A (2008) Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 57:938–944. https://doi.org/10.2337/db07-0715
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. https://doi.org/10.1126/science.1099320
Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A (2003) The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10:323–334. https://doi.org/10.1038/sj.cdd.4401148
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J, Zhang JH (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. https://doi.org/10.1016/j.pneurobio.2013.09.002
Hanschmann EM, Lonn ME, Schutte LD, Funke M, Godoy JR, Eitner S, Hudemann C, Lillig CH (2010) Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3. J Biol Chem 285:40699–40705. https://doi.org/10.1074/jbc.M110.185827
Lu J, Holmgren A (2014) The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87. https://doi.org/10.1016/j.freeradbiomed.2013.07.036
Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J (2014) Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 4:514. https://doi.org/10.3389/fimmu.2013.00514
Holmgren A (1995) Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 3:239–243
Nakamura H, Nakamura K, Yodoi J Nakamura H, Nakamura K, Yodoi J (1997) Redox regulation of cellular activation. Annu Rev Immunol 15:351–369. 15:351–369
Ramus SM, Cilensek I, Petrovic MG, Soucek M, Kruzliak P, Petrovic D (2016) Single nucleotide polymorphisms in the Trx2/TXNIP and TrxR2 genes of the mitochondrial thioredoxin antioxidant system and the risk of diabetic retinopathy in patients with Type 2 diabetes mellitus. J Diabetes Complications 30:192–198. https://doi.org/10.1016/j.jdiacomp.2015.11.021
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81371309, Zhaohui He).
Author information
Authors and Affiliations
Contributions
ZH and XC designed the project, YL and XC contributed to all experiments and to writing the manuscript. QZ, HZ, DJ, WQ, and LL helped to finish part of the experiments. JZ and XX were mainly responsible for the analysis of data and for writing the manuscript. RD helped to revise the manuscript, especially the language. All authors read and provided their approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All animal experiments were conducted according to a protocol approved by the Animal Ethics and Use Committee of Chongqing Medical University (Permit no. SCXK (Chongqing) 2007–0001).
Rights and permissions
About this article
Cite this article
Liang, Y., Che, X., Zhao, Q. et al. Thioredoxin-interacting protein mediates mitochondrion-dependent apoptosis in early brain injury after subarachnoid hemorrhage. Mol Cell Biochem 450, 149–158 (2019). https://doi.org/10.1007/s11010-018-3381-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3381-1