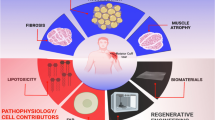
Abstract
Rotator cuff lesions (RCLs) are a common cause of shoulder pain and dysfunction. The rotator cuff tendons can degenerate and/or tear from the greater tuberosity of the humerus, which is associated with several anatomical, physiological, biochemical, and molecular changes in tendon and muscle. In this article, these pathways are critically reviewed and discussed with various management strategies of RCLs. The article also highlights the immunobiological responses following the RCL and the inherent repair mechanisms elicited by the body. The greatest difficulty in treating this pathology is that the muscle can undergo irreversible fatty infiltration in the setting of chronic tears that is associated with poor surgical outcomes. The article also investigates the key molecular pathways of the muscle homeostasis (mTOR, Rho kinase, AMPK, and Ca2+) with the energy metabolism to propose a possible mechanism for fatty infiltration. Future research is warranted to target the key players of these pathways in the management of fatty infiltration and thus RCL.






Similar content being viewed by others
References
Sher JS, Uribe JW, Posada A et al (1995) Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Jt Surg Am 77:10–15
Urwin M, Symmons D, Allison T et al (1998) Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 57:649–655
Shin KM (2011) Partial-thickness rotator cuff tears. Korean J Pain 24:69. doi:10.3344/kjp.2011.24.2.69
Lawrence RC, Helmick CG, Arnett FC et al (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41:778–799. doi:10.1002/1529-0131(199805)41:5<778:AID-ART4>3.0.CO;2-V
Matthews TJW (2006) Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Jt Surg Br 88-B:489–495. doi:10.1302/0301-620X.88B4.16845
Colvin AC, Egorova N, Harrison AK et al (2012) National trends in rotator cuff repair. J Bone Jt Surg Am 94:227–233. doi:10.2106/JBJS.J.00739
Tashjian RZ, Hollins AM, Kim H-M et al (2010) Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med 38:2435–2442. doi:10.1177/0363546510382835
Killian ML, Cavinatto L, Galatz LM, Thomopoulos S (2012) Recent advances in shoulder research. Arthritis Res Ther 14:214. doi:10.1186/ar3846
Fuchs B, Weishaupt D, Zanetti M et al (1999) Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elb Surg 8:599–605
Shimizu T, Itoi E, Minagawa H et al (2002) Atrophy of the rotator cuff muscles and site of cuff tears. Acta Orthop Scand 73:40–43. doi:10.1080/000164702317281387
Gimbel JA, Van Kleunen JP, Lake SP et al (2007) The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech 40:561–568. doi:10.1016/j.jbiomech.2006.02.010
Kim HM, Galatz LM, Lim C et al (2012) The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elb Surg 21:847–858. doi:10.1016/j.jse.2011.05.004
Rowshan K, Hadley S, Pham K et al (2010) Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Jt Surg Am 92:2270–2278. doi:10.2106/JBJS.I.00812
Brand RA (2008) Surgical anatomy of the rotator cuff and the natural history of degenerative periarthritis: Anthony F. DePalma MD, FACS. Clin Orthop 466:543–551. doi:10.1007/s11999-007-0103-5
Manske RC, Prohaska D (2008) Diagnosis and management of adhesive capsulitis. Curr Rev Musculoskelet Med 1:180–189. doi:10.1007/s12178-008-9031-6
Hodgson RJ, O’Connor PJ, Hensor EMA et al (2012) Contrast-enhanced MRI of the subdeltoid, subacromial bursa in painful and painless rotator cuff tears. Br J Radiol 85:1482–1487. doi:10.1259/bjr/45423226
Ozaki J, Fujimoto S, Nakagawa Y et al (1988) Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Jt Surg Am 70:1224–1230
Nho SJ, Yadav H, Shindle MK, Macgillivray JD (2008) Rotator cuff degeneration: etiology and pathogenesis. Am J Sports Med 36:987–993. doi:10.1177/0363546508317344
Barr KP (2004) Rotator cuff disease. Phys Med Rehabil Clin N Am 15:475–491. doi:10.1016/j.pmr.2004.03.002
Harvie P, Ostlere SJ, Teh J et al (2004) Genetic influences in the aetiology of tears of the rotator cuff. J Bone Jt Surg 86:696–700. doi:10.1302/0301-620X.86B5.14747
Hashimoto T, Nobuhara K, Hamada T (2003) Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop. doi:10.1097/01.blo.0000092974.12414.22
Kannus P, Józsa L (1991) Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Jt Surg Am 73:1507–1525
Nirschl RP (1989) Rotator cuff tendinitis: basic concepts of pathoetiology. Instr Course Lect 38:439–445
Bishay V, Gallo RA (2013) The evaluation and treatment of rotator cuff pathology. Prim Care Clin Off Pract 40:889–910. doi:10.1016/j.pop.2013.08.006
Bartolozzi A, Andreychik D, Ahmad S (1994) Determinants of outcome in the treatment of rotator cuff disease. Clin Orthop 308:90–97
Kim Y-S, Bigliani LU, Fujisawa M et al (2006) Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J Orthop Res 24:1756–1764. doi:10.1002/jor.20197
Scibek JS, Mell AG, Downie BK et al (2008) Shoulder kinematics in patients with full-thickness rotator cuff tears after a subacromial injection. J Shoulder Elb Surg 17:172–181. doi:10.1016/j.jse.2007.05.010
Cordasco FA, Chen NC, Backus SI et al (2010) Subacromial injection improves deltoid firing in subjects with large rotator cuff tears. HSS J 6:30–36. doi:10.1007/s11420-009-9127-6
Tillander B, Franzén LE, Karlsson MH, Norlin R (1999) Effect of steroid injections on the rotator cuff: an experimental study in rats. J Shoulder Elb Surg 8:271–274. doi:10.1016/S1058-2746(99)90141-6
Akpinar S, Hersekli MA, Demirors H et al (2002) Effects of methylprednisolone and betamethasone injections on the rotator cuff: an experimental study in rats. Adv Ther 19:194–201. doi:10.1007/BF02848695
Naredo E, Cabero F, Beneyto P et al (2004) A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol 31:308–314
Li X (2013) Physical therapy and rehabilitation after rotator cuff repair: a review of current concepts. Int J Phys Med Rehabil. doi:10.4172/2329-9096.1000142
Levy O, Mullett H, Roberts S, Copeland S (2008) The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elb Surg 17:863–870. doi:10.1016/j.jse.2008.04.005
Lombardi I, Magri AG, Fleury AM et al (2008) Progressive resistance training in patients with shoulder impingement syndrome: a randomized controlled trial. Arthritis Rheum 59:615–622. doi:10.1002/art.23576
Kjaer M, Magnusson P, Krogsgaard M et al (2006) Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208:445–450. doi:10.1111/j.1469-7580.2006.00549.x
Białoszewski D, Zaborowski G (2011) Usefulness of manual therapy in the rehabilitation of patients with chronic rotator cuff injuries. Preliminary report. Ortop Traumatol Rehabil 13:9–20
Eyigor C, Eyigor S, Kivilcim Korkmaz O (2010) Are intra-articular corticosteroid injections better than conventional TENS in treatment of rotator cuff tendinitis in the short run? A randomized study. Eur J Phys Rehabil Med 46:315–324
Seida JC (2010) Systematic review: nonoperative and operative treatments for rotator cuff tears. Ann Intern Med 153:246. doi:10.7326/0003-4819-153-4-201008170-00263
van der Zwaal P, Thomassen BJW, Nieuwenhuijse MJ et al (2013) Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthrosc J Arthrosc Relat Surg 29:266–273. doi:10.1016/j.arthro.2012.08.022
Kasten P, Keil C, Grieser T et al (2011) Prospective randomised comparison of arthroscopic versus mini-open rotator cuff repair of the supraspinatus tendon. Int Orthop 35:1663–1670. doi:10.1007/s00264-011-1262-2
Kobayashi M, Itoi E, Minagawa H et al (2006) Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elb Surg 15:371–377. doi:10.1016/j.jse.2005.09.003
Randelli P, Randelli F, Ragone V et al (2014) Regenerative medicine in rotator cuff injuries. Biomed Res Int 2014:1–9. doi:10.1155/2014/129515
Encalada-Diaz I, Cole BJ, MacGillivray JD et al (2011) Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Shoulder Elb Surg 20:788–794. doi:10.1016/j.jse.2010.08.013
Finosh GT, Jayabalan M (2015) Hybrid amphiphilic bimodal hydrogels having mechanical and biological recognition characteristics for cardiac tissue engineering. RSC Adv 5:38183–38201. doi:10.1039/C5RA04448K
Gnanaprakasam Thankam F, Muthu J (2014) Alginate based hybrid copolymer hydrogels—influence of pore morphology on cell–material interaction. Carbohydr Polym 112:235–244. doi:10.1016/j.carbpol.2014.05.083
Finosh GT, Jayabalan M, Vandana S, Raghu KG (2015) Hybrid alginate–polyester bimodal network hydrogel for tissue engineering—influence of structured water on long-term cellular growth. Colloids Surf B. doi:10.1016/j.colsurfb.2015.03.020
Gnanaprakasam Thankam F, Muthu J, Sankar V, Kozhiparambil Gopal R (2013) Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf B 107:137–145. doi:10.1016/j.colsurfb.2013.01.069
Montgomery SR, Petrigliano FA, Gamradt SC (2011) Biologic augmentation of rotator cuff repair. Curr Rev Musculoskelet Med 4:221–230. doi:10.1007/s12178-011-9095-6
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147. doi:10.1126/science.284.5411.143
Bi Y, Ehirchiou D, Kilts TM et al (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227. doi:10.1038/nm1630
Tsai C-C, Huang T-F, Ma H-L et al (2013) Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant 22:413–422. doi:10.3727/096368912X656090
Randelli P, Conforti E, Piccoli M et al (2013) Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity. Am J Sports Med 41:1653–1664. doi:10.1177/0363546512473572
Utsunomiya H, Uchida S, Sekiya I et al (2013) Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med 41:657–668. doi:10.1177/0363546512473269
Meyer AW (1922) Further observations upon use-destruction in joints. J Bone Jt Surg Am 4:491–511
Lindblom K (1939) On pathogenesis of ruptures of the tendon aponeurosis of the shoulder joint. Acta Radiol 20:563–577. doi:10.3109/00016923909174877
Codman EA, Akerson IB (1931) The pathology associated with rupture of the supraspinatus tendon. Ann Surg 93:348–359. doi:10.1097/00000658-193101000-00043
Neer CS II, Poppen NK (1987) Supraspinatus outlet. Orthop Trans 11:234
Balke M, Schmidt C, Dedy N et al (2013) Correlation of acromial morphology with impingement syndrome and rotator cuff tears. Acta Orthop 84:178–183. doi:10.3109/17453674.2013.773413
McMaster WC, Troup J (1993) A survey of interfering shoulder pain in United States competitive swimmers. Am J Sports Med 21:67–70
Galatz LM, Silva MJ, Rothermich SY et al (2006) Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Jt Surg Am 88:2027–2034. doi:10.2106/JBJS.E.00899
Mallon WJ, Misamore G, Snead DS, Denton P (2004) The impact of preoperative smoking habits on the results of rotator cuff repair. J Shoulder Elb Surg 13:129–132. doi:10.1016/S1058274603002805
Xu Y, Murrell GAC (2008) The basic science of tendinopathy. Clin Orthop 466:1528–1538. doi:10.1007/s11999-008-0286-4
Codman EA (1934) The shoulder: rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. R.E. Kreiger, Florida
Aydin N, Tok O, Görgün B (2014) Rotator cuff tear arthropathy: pathophysiology, diagnosis and treatment. Orthop Muscular Syst 3:1000159
Macaulay AA, Greiwe RM, Bigliani LU (2010) Rotator cuff deficient arthritis of the glenohumeral joint. Clin Orthop Surg 2:196. doi:10.4055/cios.2010.2.4.196
De Giorgi S, Saracino M, Castagna A (2013) Degenerative disease in rotator cuff tears: What are the biochemical and histological changes? Joints 2:26–28
Tashjian RZ (2012) Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 31:589–604. doi:10.1016/j.csm.2012.07.001
Hampson K, Forsyth NR, El Haj A, Maffulli N (2008) Tendon tissue engineering. Top Tissue Eng 4:1–21
Fukuta S, Oyama M, Kavalkovich K et al (1998) Identification of types II, IX and X collagens at the insertion site of the bovine Achilles tendon. Matrix Biol 17:65–73. doi:10.1016/S0945-053X(98)90125-1
Wang JH-C (2006) Mechanobiology of tendon. J Biomech 39:1563–1582. doi:10.1016/j.jbiomech.2005.05.011
Vogel KG, Koob TJ (1989) Structural specialization in tendons under compression. In: Friedlander M, Jeon KW (eds) International review of cytology. Academic Press, Cambridge, pp 267–293
Pins GD, Christiansen DL, Patel R, Silver FH (1997) Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J 73:2164–2172. doi:10.1016/S0006-3495(97)78247-X
Sharma P, Maffulli N (2005) Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 6:181–190
Banes AJ, Link GW, Bevin AG et al (1988) Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res 6:73–82. doi:10.1002/jor.1100060110
Sun Y, Berger EJ, Zhao C et al (2006) Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res 47:215–221. doi:10.1080/03008200600846754
Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10:312–320
Kjær M, Langberg H, Heinemeier K et al (2009) From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 19:500–510. doi:10.1111/j.1600-0838.2009.00986.x
Chaudhury S, Dines JS, Delos D et al (2012) Role of fatty infiltration in the pathophysiology and outcomes of rotator cuff tears. Arthritis Care Res 64:76–82. doi:10.1002/acr.20552
Apostolakos J, Durant TJS, Dwyer CR et al (2014) The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J 4:333–342
Lui P, Zhang P, Chan K, Qin L (2010) Biology and augmentation of tendon–bone insertion repair. J Orthop Surg 5:59. doi:10.1186/1749-799X-5-59
Thomopoulos S, Genin GM, Galatz LM (2010) The development and morphogenesis of the tendon-to-bone insertion—what development can teach us about healing. J Musculoskelet Neuronal Interact 10:35–45
Dean BJF, Franklin SL, Carr AJ (2012) A systematic review of the histological and molecular changes in rotator cuff disease. Bone Jt Res 1:158–166. doi:10.1302/2046-3758.17.2000115
Tillander B, Franzén L, Norlin R (2002) Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res 20:1358–1364. doi:10.1016/S0736-0266(02)00057-8
Mannava S, Plate JF, Tuohy CJ et al (2013) The science of rotator cuff tears: translating animal models to clinical recommendations using simulation analysis. Knee Surg Sports Traumatol Arthrosc 21:1610–1619. doi:10.1007/s00167-012-2145-9
Tuoheti Y, Itoi E, Pradhan RL et al (2005) Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elb Surg 14:535–541. doi:10.1016/j.jse.2005.01.001
Maffulli N, Renstrom P, Leadbetter WB (2005) Tendon injuries: basic science and clinical medicine. Springer, London
Bunker T (2002) Rotator cuff disease. Curr Orthop 16:223–233. doi:10.1054/cuor.2002.0257
Hashimoto T, Nobuhara K, Hamada T (2003) Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop 111–120. doi:10.1097/01.blo.0000092974.12414.22
Chuen FS, Chuk CY, Ping WY et al (2004) Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem 52:1151–1157. doi:10.1369/jhc.3A6232.2004
Kajikawa Y, Morihara T, Watanabe N et al (2007) GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J Cell Physiol 210:684–691. doi:10.1002/jcp.20876
James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg 33:102–112. doi:10.1016/j.jhsa.2007.09.007
Mora MV (2015) Stem cell therapy in the management of shoulder rotator cuff disorders. World J Stem Cells 7:691. doi:10.4252/wjsc.v7.i4.691
Wang JH-C, Iosifidis MI, Fu FH (2006) Biomechanical basis for tendinopathy. Clin Orthop 443:320–332. doi:10.1097/01.blo.0000195927.81845.46
Bedi A, Maak T, Walsh C et al (2012) Cytokines in rotator cuff degeneration and repair. J Shoulder Elb Surg 21:218–227. doi:10.1016/j.jse.2011.09.020
Evans CH (1999) Cytokines and the role they play in the healing of ligaments and tendons. Sports Med Auckl NZ 28:71–76
Millar NL, Wei AQ, Molloy TJ et al (2009) Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Jt Surg Br 91-B:417–424. doi:10.1302/0301-620X.91B3.21652
Gelberman RH, Steinberg D, Amiel D, Akeson W (1991) Fibroblast chemotaxis after tendon repair. J Hand Surg 16:686–693. doi:10.1016/0363-5023(91)90195-H
Voleti PB, Buckley MR, Soslowsky LJ (2012) Tendon healing: repair and regeneration. Annu Rev Biomed Eng 14:47–71. doi:10.1146/annurev-bioeng-071811-150122
Docheva D, Müller SA, Majewski M, Evans CH (2015) Biologics for tendon repair. Adv Drug Deliv Rev 84:222–239. doi:10.1016/j.addr.2014.11.015
Maffulli N, Longo UG (2008) How do eccentric exercises work in tendinopathy? Rheumatol Oxf Engl 47:1444–1445. doi:10.1093/rheumatology/ken337
Kader D, Saxena A, Movin T, Maffulli N (2002) Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med 36:239–249
Camargo PR (2014) Eccentric training as a new approach for rotator cuff tendinopathy: review and perspectives. World J Orthop 5:634. doi:10.5312/wjo.v5.i5.634
Tang JB, Cao Y, Zhu B et al (2008) Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. An in vivo study. J Bone Jt Surg Am 90:1078–1089. doi:10.2106/JBJS.F.01188
Dahlgren LA, Mohammed HO, Nixon AJ (2005) Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res 23:84–92. doi:10.1016/j.orthres.2004.05.007
Lu H (2006) Low-intensity pulsed ultrasound accelerates bone–tendon junction healing: a partial patellectomy model in rabbits. Am J Sports Med 34:1287–1296. doi:10.1177/0363546506286788
Seeherman HJ (2008) rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Jt Surg Am 90:2206. doi:10.2106/JBJS.G.00742
Silvana DE, Giorgi MS (2014) Degenerative disease in rotator cuff tears: what are the biochemical and histological changes? Joints 2:26–28
Magra M, Maffulli N (2005) Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med 39:789–791. doi:10.1136/bjsm.2005.017855
Arnoczky SP, Lavagnino M, Egerbacher M et al (2007) Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med 35:763–769. doi:10.1177/0363546506296043
Gardner K, Arnoczky SP, Caballero O, Lavagnino M (2008) The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: an in vitro experimental study. Disabil Rehabil 30:1523–1529. doi:10.1080/09638280701785395
Osawa T, Shinozaki T, Takagishi K (2005) Multivariate analysis of biochemical markers in synovial fluid from the shoulder joint for diagnosis of rotator cuff tears. Rheumatol Int 25:436–441. doi:10.1007/s00296-004-0509-2
Choi H-R, Kondo S, Hirose K et al (2002) Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res 20:927–933. doi:10.1016/S0736-0266(02)00016-5
Pearce WH, Shively VP (2006) Abdominal aortic aneurysm as a complex multifactorial disease: interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of MMPs. Ann N Y Acad Sci 1085:117–132. doi:10.1196/annals.1383.025
Franceschi F, Longo UG, Ruzzini L et al (2008) Circulating substance P levels and shoulder joint contracture after arthroscopic repair of the rotator cuff. Br J Sports Med 42:742–745. doi:10.1136/bjsm.2007.040931
Pasternak B, Missios A, Askendal A et al (2007) Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Acta Orthop 78:680–686. doi:10.1080/17453670710014392
Rees JD, Stride M, Scott A (2014) Tendons—time to revisit inflammation. Br J Sports Med 48:1553–1557. doi:10.1136/bjsports-2012-091957
Premdas J, Tang JB, Warner JP et al (2001) The presence of smooth muscle actin in fibroblasts in the torn human rotator cuff. J Orthop Res Off Publ Orthop Res Soc 19:221–228. doi:10.1016/S0736-0266(00)90011-1
Luan T, Liu X, Easley JT et al (2015) Muscle atrophy and fatty infiltration after an acute rotator cuff repair in a sheep model. Muscles Ligaments Tendons J 5:106–112
Liu X, Joshi SK, Samagh SP et al (2012) Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res Off Publ Orthop Res Soc 30:1440–1446. doi:10.1002/jor.22096
Rosen ED, Sarraf P, Troy AE et al (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617
Fajas L, Schoonjans K, Gelman L et al (1999) Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol 19:5495–5503
Joshi SK, Liu X, Samagh SP et al (2013) mTOR regulates fatty infiltration through SREBP-1 and PPARγ after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res Off Publ Orthop Res Soc 31:724–730. doi:10.1002/jor.22254
Porstmann T, Santos CR, Griffiths B et al (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8:224–236. doi:10.1016/j.cmet.2008.07.007
Blanchard P-G, Festuccia WT, Houde VP et al (2012) Major involvement of mTOR in the PPAR-induced stimulation of adipose tissue lipid uptake and fat accretion. J Lipid Res 53:1117–1125. doi:10.1194/jlr.M021485
Lai K-MV, Gonzalez M, Poueymirou WT et al (2004) Conditional activation of Akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304. doi:10.1128/MCB.24.21.9295-9304.2004
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293. doi:10.1016/j.cell.2012.03.017
Le Bacquer O, Petroulakis E, Paglialunga S et al (2007) Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Investig 117:387–396. doi:10.1172/JCI29528
Mounier R, Lantier L, Leclerc J et al (2011) Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle Georget Tex 10:2640–2646
Düvel K, Yecies JL, Menon S et al (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183. doi:10.1016/j.molcel.2010.06.022
Wang BT, Ducker GS, Barczak AJ et al (2011) The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc Natl Acad Sci USA 108:15201–15206. doi:10.1073/pnas.1103746108
Peterson TR, Sengupta SS, Harris TE et al (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146:408–420. doi:10.1016/j.cell.2011.06.034
Zhang HH, Huang J, Düvel K et al (2009) Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. doi:10.1371/journal.pone.0006189
Tee AR, Manning BD, Roux PP et al (2003) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13:1259–1268
Ma L, Chen Z, Erdjument-Bromage H et al (2005) Phosphorylation and functional inactivation of TSC2 by Erk: implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179–193. doi:10.1016/j.cell.2005.02.031
Lee DF, Kuo HP, Chen CT et al (2007) IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130:440–455. doi:10.1016/j.cell.2007.05.058
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226. doi:10.1016/j.molcel.2008.03.003
Viollet B, Lantier L, Devin-Leclerc J et al (2009) Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front Biosci Landmark Ed 14:3380–3400
Liu L, Cash TP, Jones RG et al (2006) Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell 21:521–531. doi:10.1016/j.molcel.2006.01.010
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484. doi:10.1016/j.cell.2006.01.016
Amano M, Nakayama M, Kaibuchi K (2010) Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67:545–554. doi:10.1002/cm.20472
Leung T, Chen XQ, Manser E, Lim L (1996) The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16:5313–5327
Wang Y, Zheng XR, Riddick N et al (2009) ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res 104:531–540. doi:10.1161/CIRCRESAHA.108.188524
Yamaguchi H, Kasa M, Amano M et al (2006) Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Struct Lond Engl 1993 14:589–600. doi:10.1016/j.str.2005.11.024
Ishizaki T, Naito M, Fujisawa K et al (1997) p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 404:118–124
Araki S, Ito M, Kureishi Y et al (2001) Arachidonic acid-induced Ca2+ sensitization of smooth muscle contraction through activation of Rho-kinase. Pflüg Arch 441:596–603. doi:10.1007/s004240000462
Ito M, Nakano T, Erdodi F, Hartshorne DJ (2004) Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259:197–209
Kimura K, Ito M, Amano M et al (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248
Kamm KE, Stull JT (2001) Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276:4527–4530. doi:10.1074/jbc.R000028200
Yoshioka K, Sugimoto N, Takuwa N, Takuwa Y (2007) Essential role for class II phosphoinositide 3-kinase alpha-isoform in Ca2+-induced, Rho- and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Mol Pharmacol 71:912–920. doi:10.1124/mol.106.032599
Melis B, Nemoz C, Walch G (2009) Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res 95:319–324. doi:10.1016/j.otsr.2009.05.001
Goutallier D, Postel JM, Bernageau J et al (1994) Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop 304:78–83
Williams DE, Sturgeon CM, Roberge M, Andersen RJ (2007) Nigricanosides A and B, antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in Dominica. J Am Chem Soc 129:5822–5823. doi:10.1021/ja0715187
Schaefer O, Winterer J, Lohrmann C et al (2002) Magnetic resonance imaging for supraspinatus muscle atrophy after cuff repair. Clin Orthop 403:93–99
Abtahi AM (2015) Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop 6:211. doi:10.5312/wjo.v6.i2.211
Goutallier D, Postel J-M, Gleyze P et al (2003) Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elb Surg 12:550–554. doi:10.1016/S1058274603002118
Melis B, Wall B, Walch G (2010) Natural history of infraspinatus fatty infiltration in rotator cuff tears. J Shoulder Elb Surg 19:757–763. doi:10.1016/j.jse.2009.12.002
Karampinos DC, Baum T, Nardo L et al (2012) Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging 35:899–907. doi:10.1002/jmri.23512
Coen PM, Goodpaster BH (2012) Role of intramyocellular lipids in human health. Trends Endocrinol Metab 23:391–398. doi:10.1016/j.tem.2012.05.009
Gerber C, Fuchs B, Hodler J (2000) The results of repair of massive tears of the rotator cuff. J Bone Jt Surg Am 82:505–515
Addison O, Marcus RL, LaStayo PC, Ryan AS (2014) Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014:1–11. doi:10.1155/2014/309570
Frey E, Regenfelder F, Sussmann P et al (2009) Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res 27:504–509. doi:10.1002/jor.20695
Itoigawa Y, Kishimoto KN, Sano H et al (2011) Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res 29:861–866. doi:10.1002/jor.21317
Schmutz S, Fuchs T, Regenfelder F et al (2009) Expression of atrophy mRNA relates to tendon tear size in supraspinatus muscle. Clin Orthop 467:457–464. doi:10.1007/s11999-008-0565-0
Acknowledgments
This work was supported by Research Grants R01 HL116042 and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA and Haddix Grant to MF Dilisio. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have read the journal’s policy on disclosure of potential conflicts of interest and the authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. All authors declare that there is no conflict. No writing assistance was utilized in the production of this manuscript.
Rights and permissions
About this article
Cite this article
Thankam, F.G., Dilisio, M.F. & Agrawal, D.K. Immunobiological factors aggravating the fatty infiltration on tendons and muscles in rotator cuff lesions. Mol Cell Biochem 417, 17–33 (2016). https://doi.org/10.1007/s11010-016-2710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2710-5