Abstract
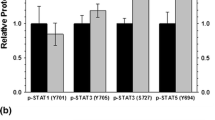
During hibernation, the metabolic rate of thirteen-lined ground squirrels (Ictidomys tridecemlineatus) can drop to <5 % of normal resting rate at 37 °C, core body temperature can decrease to as low as 1–5 °C, and heart rate can fall from 350–400 to 5–10 bpm. Energy saved by hibernating allows squirrels to survive the winter when food is scarce, and living off lipid reserves in white adipose tissue (WAT) is crucial. While hibernating, some energy must be used to cope with conditions that would normally be damaging for mammals (e.g., low core body temperatures, ischemia) and could induce cell death via apoptosis. Cell survival is largely dependent on the relative amounts and activities of pro- and anti-apoptotic Bcl-2 family proteins. The present study analyzed how anti-apoptotic proteins respond to protect WAT cells during hibernation. Relative levels of several anti-apoptotic proteins were quantified in WAT via immunoblotting over six time points of the torpor-arousal cycle. These included anti-apoptotic Bcl-2 family members Bcl-2, Bcl-xL, and Mcl-l, as well as caspase inhibitors x-IAP and c-IAP. Changes in the relative protein levels and/or phosphorylation levels were also observed for various regulators of apoptosis (p-JAKs, p-STATs, SOCS, and PIAS). Mcl-1 and x-IAP protein levels increased whereas Bcl-xL, Bcl-2, and c-IAP protein/phosphorylation levels decreased signifying important roles for certain Bcl-2 family members in cell survival over the torpor-arousal cycle. Importantly, the relative phosphorylation of selected STAT proteins increased, suggesting a mechanism for Bcl-2 family activation. These results suggest that an increase in WAT cytoprotective mechanisms supports survival efforts during hibernation.










Similar content being viewed by others
References
Storey KB, Heldmaier G, Rider MH (2010) Mammalian hibernation: Physiology, cell signaling, and gene control on metabolic rate depression. In: Lubzens E (ed) Dormancy and resistance in harsh environments. Springer-Verlag, Berlin, Heidelberg, pp 227–252
Storey KB (2010) Out cold: biochemical regulation of mammalian hibernation—a mini-review. Gerontology 56:220–230. doi:10.1159/000228829
Storey KB, Storey JM (2004) Mammalian hibernation: biochemical adaptation and gene expression. In: Storey KB (ed) Functional metabolism: regulation and adaptation. Wiley Inc., Hoboken, pp 443–472
Wang LCH, Lee TF (1996) Torpor and hibernation in mammals: metabolic, physiological, and biochemical adaptations. In: Handbook of physiology—environmental physiology, pp 507–532
Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL (2013) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527. doi:10.1086/673092
Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE (2010) Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 285:3428–3438. doi:10.1074/jbc.M109.074252
Gullicksen PS, Hausman DB, Dean RG, Hartzell DL, Baile CA (2003) Adipose tissue cellularity and apoptosis after intracerebroventricular injections of leptin and 21 days of recovery in rats. Int J Obes Relat Metab Disord 27:302–312. doi:10.1038/sj.ijo.0802205
Herold C, Rennekampff HO, Engeli S (2013) Apoptotic pathways in adipose tissue. Apoptosis 18:911–916. doi:10.1007/s10495-013-0848-0
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241. doi:10.1038/nrm2312
Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR (2005) Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science 310:66–67. doi:10.1126/science.1117105
Labi V, Erlacher M (2015) How cell death shapes cancer. Cell death 6:e1675. doi:10.1038/cddis.2015.20
Tinahones FJ, Coín Aragüez L, Murri M, Oliva Olivera W, Mayas Torres MD, Barbarroja N, Gomez Huelgas R, Malagón MM, El Bekay R (2013) Caspase induction and BCL2 inhibition in human adipose tissue: a potential relationship with insulin signaling alteration. Diabetes Care 36:513–521. doi:10.2337/dc12-0194
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR (2010) The BCL-2 family reunion. Mol Cell 37:299–310. doi:10.1016/j.molcel.2010.01.025
Gyrd-Hansen M, Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer 10:561–574. doi:10.1038/nrc2979
Perez HL, Chaudhry C, Emanuel SL, Fanslau C, Fargnoli J, Gan J, Kim KS, Lei M, Naglich JG, Traeger SC, Vuppugalla R, Wei DD, Vite GD, Talbott RL, Borzilleri RM (2015) Discovery of potent heterodimeric antagonists of inhibitor of apoptosis proteins (IAPs) with sustained antitumor activity. J Med Chem 58:1556–1562. doi:10.1021/jm501482t
Al Zaid Siddiquee K, Turkson J (2008) STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 18:254–267. doi:10.1038/cr.2008.18
Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BKH, Hui KM, Sethi G (2013) Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta: Rev Cancer 1835:46–60. doi:10.1016/j.bbcan.2012.10.002
Stephanou A, Latchman DS (2003) STAT-1: a novel regulator of apoptosis. Int J Exp Pathol 84:239–244. doi:10.1111/j.0959-9673.2003.00363.x
Kiu H, Nicholson SE (2012) Biology and significance of the JAK/STAT signalling pathways. Growth Factors 30:88–106. doi:10.3109/08977194.2012.660936
Shuai K, Liu B (2005) Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol 5:593–605. doi:10.1038/nri1667
Alvarez JV, Frank DA (2004) Genome-wide analysis of STAT target genes: elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol Ther 3:1045–1050. doi:10.4161/cbt.3.11.1172
Koch V, Staab J, Ruppert V, Meyer T (2012) Two glutamic acid residues in the DNA-binding domain are engaged in the release of STAT1 dimers from DNA. BMC Cell Biol 13:22. doi:10.1186/1471-2121-13-22
Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BKH, Sethi G, Bishayee A (2014) Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 1845:136–154. doi:10.1016/j.bbcan.2013.12.005
Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR (1997) Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278:1630–1632. doi:10.1126/science.278.5343.1630
Yu H, Jove R (2004) The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4:97–105. doi:10.1038/nrc1275
Furqan M, Mukhi N, Lee B, Liu D (2013) Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 1:1–5. doi:10.1186/2050-7771-1-5
Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A (2011) Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol 31:980–985. doi:10.1161/ATVBAHA.110.207464
Valentino L, Pierre J (2006) JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol 71:713–721. doi:10.1016/j.bcp.2005.12.017
Rouble AN, Hefler J, Mamady H, Storey KB, Tessier SN (2013) Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. Peer J 1:e29. doi:10.7717/peerj.29
McMullen DC, Hallenbeck JM (2010) Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Comp Physiol B Biochem Syst Environ Physiol 180:927–934. doi:10.1007/s00360-010-0468-8
Luu BE, Tessier SN, Duford DL, Storey KB (2015) The regulation of troponins I, C and ANP by GATA4 and Nk2–5 in heart of hibernating thirteen-lined ground squirrels. PLoS ONE 10:e0117747. doi:10.1371/journal.pone.0117747
Rouble AN, Tessier SN, Storey KB (2014) Characterization of adipocyte stress response pathways during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem 393:271–282. doi:10.1007/s11010-014-2070-y
Storey KB (2015) Regulation of hypometabolism: insights into epigenetic controls. J Exp Biol 218:150–159. doi:10.1242/jeb.106369
Boyer BB, Barnes BM (1999) Molecular and metabolic aspects of mammalian hibernation. Bioscience 49:713–724. doi:10.2307/1313595
Carpenter RL, Lo HW (2014) STAT3 target genes relevant to human cancers. Cancers 6:897–925. doi:10.3390/cancers6020897
Bah N, Maillet L, Ryan J, Dubreil S, Gautier F, Letai A, Juin P, Barillé-Nion S (2014) Bcl-xL controls a switch between cell death modes during mitotic arrest. Cell Death Dis 5:e1291. doi:10.1038/cddis.2014.251
Blagosklonny MV (2001) Unwinding the loop of Bcl-2 phosphorylation. Leuk Off J Leuk Soc Am Leuk Res Fund UK 15:869–874. doi:10.1038/sj.leu.2402134
De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, Palamara AT, Russo T, Garaci E, Cozzolino F (2006) Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem 281:21353–21361. doi:10.1074/jbc.M511052200
D’Amours D, Sallmann FR, Dixit VM, Poirier GG (2001) Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 114:3771–3778
Zhao J, Xin M, Wang T, Zhang Y, Deng X (2009) Nicotine enhances the antiapoptotic function of Mcl-1 through phosphorylation. Mol Cancer Res 7:1954–1961. doi:10.1158/1541-7786.MCR-09-0304
Ren H, Koo J, Guan B, Yue P, Deng X, Chen M, Khuri FR, Sun S-Y (2013) The E3 ubiquitin ligases β-TrCP and FBXW7 cooperatively mediates GSK3-dependent Mcl-1 degradation induced by the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer 12:146. doi:10.1186/1476-4598-12-146
Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR (2006) Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21:749–760. doi:10.1016/j.molcel.2006.02.009
Satoh JI, Tabunoki H (2013) A comprehensive profile of ChIP-Seq-based STAT1 target genes suggests the complexity of STAT1-mediated gene regulatory mechanisms. Gene Regul Syst Bio 2013:41–56. doi:10.4137/GRSB.S11433
Calò V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A (2003) STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 197:157–168. doi:10.1002/jcp.10364
Stephanou A (2009) JAK-STAT pathway in disease. Landes Bioscience, Austin
Rytinki MM, Kaikkonen S, Pehkonen P, Jääskeläinen T, Palvimo JJ (2009) PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci 66:3029–3041. doi:10.1007/s00018-009-0061-z
Schmidt D, Müller S (2003) PIAS/SUMO: new partners in transcriptional regulation. Cell Mol Life Sci 60:2561–2574. doi:10.1007/s00018-003-3129-1
Larsen L, Ropke C (2002) Suppressors of cytokine signalling: SOCS. Apmis 110:833–844. doi:10.1034/j.1600-0463.2002.1101201.x
Sen B, Peng S, Woods DM, Wistuba I, Bell D, El-Nagar A, Lai SY, Johnson FM (2012) STAT5A-mediated SOCS2 expression regulates Jak2 and STAT3 activity following c-Src inhibition in head and neck squamous carcinoma. Clin Cancer Res 18:127–139. doi:10.1158/1078-0432.CCR-11-1889
Basham B, Sathe M, Grein J, McClanahan T, D’Andrea A, Lees E, Rascle A (2008) In vivo identification of novel STAT5 target genes. Nucleic Acids Res 36:3802–3818. doi:10.1093/nar/gkn271
Richard AJ, Stephens JM (2011) Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol Metab 22:325–332. doi:10.1016/j.tem.2011.03.007
Acknowledgments
Thanks to J.M. Storey for editorial review of this manuscript. This work was supported by a Discovery Grant (#6793) from the Natural Sciences and Engineering Research Council of Canada. KBS holds the Canada Research Chair in Molecular Physiology, BEL holds an NSERC Canada Graduate Scholarship, and SML held an NSERC Undergraduate Summer Research Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logan, S.M., Luu, B.E. & Storey, K.B. Turn down genes for WAT? Activation of anti-apoptosis pathways protects white adipose tissue in metabolically depressed thirteen-lined ground squirrels. Mol Cell Biochem 416, 47–62 (2016). https://doi.org/10.1007/s11010-016-2695-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2695-0