Abstract
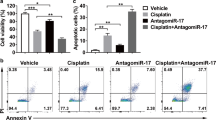
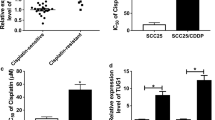
Chemoresistance is a challenge for clinician in management of tongue cancer. Therefore, it is necessary to explore alternative therapeutic methods to overcome drug resistance. miRNAs are endogenous −22nt RNAs that play important regulatory roles by targeting mRNAs. miR-21, an essential oncogenic molecule, is associated with chemosensitivity of several human cancer cells to anticancer agents. In this study, we investigated the effects and molecular mechanisms of miR-21 in chemosensitivity of tongue squamous cell carcinoma cells (TSCC) to cisplatin. miR-21 expression was detected in tongue cancer tissue using RT-PCR and PDCD4 protein expression was measured using immunohistochemistry. miR-21 and(or) PDCD4 depleted cell lines were generated using miR-21 inhibitor and(or) siRNA. The viabilities of treated cells were analyzed using MTT assay. RT-PCR was used to detect miR-21 expression and immunoblotting was used to detect protein levels. Cell cycle and apoptosis were analyzed using propidium iodide (PI) staining and Annexin V/PI staining, respectively. The expression of miR-21 in tumorous tissue was significantly higher compared with adjacent normal tissue and loss of PDCD4 expression was observed in TSCCs. Transfection of miR-21 inhibitor induced sensitivity of TSCC cells (Tca8113 and CAL-27) to cisplatin. TSCC cells transfected with PDCD4 siRNA became more resistant to cisplatin therapy. We found an increase PDCD4 protein level following the transfection of miR-21 inhibitor using Western blot analysis. In addition, the enhanced growth-inhibitory effect by miR-21 inhibitor was weakened after the addition of PDCD4 siRNA. Suppression of miR-21 or PDCD4 could significantly promote or reduce cisplatin-induced apoptosis, respectively. Our data suggest that miR-21 could modulate chemosensitivity of TSCC cells to cisplatin by targeting PDCD4, and miR-21 may serve as a potential target for TSCC therapy.











Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. doi:10.3322/caac.20138
Gibson MK, Forastiere AA (2006) Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol 7:565–574. doi:10.1016/S1470-2045(06)70757-4
O’Neill VJ, Twelves CJ (2002) Oral cancer treatment: developments in chemotherapy and beyond. Br J Cancer 87:933–937
Stewart DJ (2007) Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 63:12–31. doi:10.1016/j.critrevonc.2007.02.001
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi:10.1016/s0092-8674(04)00045-5
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531. doi:10.1038/nrg1379
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302:1–12. doi:10.1016/j.ydbio.2006.08.028
Blower PE, Chung J-H, Verducci JS, Lin S, Park J-K, Dai Z, Liu C-G, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W (2008) MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther 7:1–9. doi:10.1158/1535-7163.mct-07-0573
Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103:2257–2261. doi:10.1073/pnas.0510565103
Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E (2009) MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res 15:3998–4008. doi:10.1158/1078-0432.CCR-08-3053
Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, Nuovo GJ, Fishel R, Croce CM (2010) MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA 107:21098–21103. doi:10.1073/pnas.1015541107
Bai HT, Xu R, Cao ZW, Wei DL, Wang C (2011) Involvement of miR-21 in resistance to daunorubicin by regulating PTEN expression in the leukaemia K562 cell line. FEBS Lett 585:402–408. doi:10.1016/j.febslet.2010.12.027
Gong C, Yao YD, Wang Y, Liu BD, Wu W, Chen JN, Su FX, Yao HR, Song EW (2011) Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem 286:19127–19137. doi:10.1074/jbc.M110.216887
Wang W, Songlin P, Sun Y, Zhang B, Jinhui W (2012) miR-21 inhibitor sensitizes human OSCC cells to cisplatin. Mol Biol Rep 39:5481–5485. doi:10.1007/s11033-011-1350-9
Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283:1026–1033. doi:10.1074/jbc.M707224200
Asangani IA, Rasheed SAK, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H (2007) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27:2128-2136. http://www.nature.com/onc/journal/v27/n15/full/1210856a.html
Jansen AP, Camalier CE, Stark C, Colburn NH (2004) Characterization of programmed cell death 4 in multiple human cancers reveals a novel enhancer of drug sensitivity. Mol Cancer Ther 3:103–110
Zhang X, Wang X, Song X, Liu C, Shi Y, Wang Y, Afonja O, Ma C, Chen YH, Zhang L (2010) Programmed cell death 4 enhances chemosensitivity of ovarian cancer cells by activating death receptor pathway in vitro and in vivo. Cancer Sci 101:2163–2170. doi:10.1111/j.1349-7006.2010.01664.x
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
dos Reis PP, Bharadwaj RR, Machado J, MacMillan C, Pintilie M, Sukhai MA, Perez-Ordonez B, Gullane P, Irish J, Kamel-Reid S (2008) Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer 113:3169–3180. doi:10.1002/cncr.23934
Cornelissen M, Philippe J, De Sitter S, De Ridder L (2002) Annexin V expression in apoptotic peripheral blood lymphocytes: an electron microscopic evaluation. Apoptosis 7:41–47
Reis PP, Tomenson M, Cervigne NK, Machado J, Jurisica I, Pintilie M, Sukhai MA, Perez-Ordonez B, Grenman R, Gilbert RW, Gullane PJ, Irish JC, Kamel-Reid S (2010) Programmed cell death 4 loss increases tumor cell invasion and is regulated by miR-21 in oral squamous cell carcinoma. Mol Cancer 9:238. doi:10.1186/1476-4598-9-238
Tomimaru Y, Eguchi H, Nagano H, Wada H, Tomokuni A, Kobayashi S, Marubashi S, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M (2010) MicroRNA-21 induces resistance to the anti-tumour effect of interferon-α/5-fluorouracil in hepatocellular carcinoma cells. Br J Cancer 103:1617–1626
Hwang J-H, Voortman J, Giovannetti E, Steinberg SM, Leon LG, Kim Y-T, Funel N, Park JK, Kim MA, Kang GH, Kim S-W, Chiaro MD, Peters GJ, Giaccone G (2010) Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One 5:e10630. doi:10.1371/journal.pone.0010630
Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T (2006) Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 130:2113–2129. doi:10.1053/j.gastro.2006.02.057
Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A, Danesi R, Campani D, Verheul HM, Boggi U (2010) MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res 70:4528–4538. doi:10.1158/0008-5472.can-09-4467
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT, Zhang CP (2010) MicroRNAs contribute to the chemoresistance of cisplatin in tongue squamous cell carcinoma lines. Oral Oncol 46:317–322. doi:10.1016/j.oraloncology.2010.02.002
Meng FY, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133:647–658. doi:10.1053/j.gastro.2007.05.022
Zhu S, Si M-L, Wu H, Mo Y-Y (2007) MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282:14328–14336. doi:10.1074/jbc.M611393200
Bourguignon LYW, Spevak CC, Wong G, Xia WL, Gilad E (2009) Hyaluronan-CD44 interaction with protein kinase C epsilon promotes oncogenic signaling by the stem cell marker nanog and the production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem 284:26533–26546. doi:10.1074/jbc.M109.027466
Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, Ideguchi H, Yamamoto K, Matsuhashi S (2006) Involvement of programmed cell death 4 in transforming growth factor-β1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25:6101–6112
Park JK, Lee EJ, Esau C, Schmittgen TD (2009) Antisense inhibition of microRNA-21 or-221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 38:E190–E199
Wang P, Zou FD, Zhang XD, Li H, Dulak A, Tomko RJ, Lazo JS, Wang Z, Zhang L, Yu J (2009) MicroRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 69:8157–8165. doi:10.1158/0008-5472.can-09-1996
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038. http://www.nature.com/nature/journal/v465/n7301/full/nature09144.html
Sumazin P, Yang X, Chiu H-S, Chung W-J, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, Califano A (2011) An extensive microRNA-mediated network of RNA–RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147:370–381
Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP (2011) Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147:344–357. doi:10.1016/j.cell.2011.09.029
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147:358–369
Karreth Florian A, Tay Y, Perna D, Ala U, Tan Shen M, Rust Alistair G, DeNicola G, Webster Kaitlyn A, Weiss D, Perez-Mancera Pedro A, Krauthammer M, Halaban R, Provero P, Adams David J, Tuveson David A, Pandolfi Pier P (2011) In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147:382–395. doi:10.1016/j.cell.2011.09.032
Acknowledgments
This work was supported by National Natural Science Foundation of China (81272957), Specialized Research Fund for the Doctoral Program of Higher Education (20120201110063, 20130201120054), the Fundamental Research Funds for the Central Universities (XJJ2013057) and innovation project of Shaanxi province (2012KTCL03-17).
Conflict of interest
We declare that we have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaolong Wang, Ling Gao, and Wenhao Ren are co-first authors and contributed equally to this article.
Rights and permissions
About this article
Cite this article
Ren, W., Wang, X., Gao, L. et al. miR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol Cell Biochem 390, 253–262 (2014). https://doi.org/10.1007/s11010-014-1976-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-1976-8