Abstract
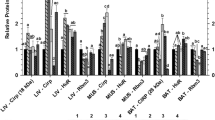
Hibernating mammals endure conditions of low body temperature and oxidative stress that would be highly injurious to humans and most other mammals. Stress conditions frequently trigger the production of molecular chaperones; in the endoplasmic reticulum the glucose-regulated protein-78 (GRP78) helps to minimize protein misfolding under stress. The present study evaluated the GRP78 response in seven organs of hibernating thirteen-lined ground squirrels, Spermophilus tridecemlineatus. Transcript levels of grp78, assessed by RT-PCR, were significantly higher (3.5- to 4.1-fold) in brown adipose tissue and brain of hibernating squirrels compared with euthermic control animals but remained low or stable in all other tissues. GRP78 protein content, assessed by Western blotting, was also elevated in brown adipose and brain during hibernation by 1.4–1.6 fold. A 2490 bp cDNA sequence was retrieved that contained the full length open reading frame of ground squirrel grp78 and the translated protein sequence of 654 amino acids shared 98–99% identity with GRP78 from other mammalian sources. Selected specific amino acid substitutions were found in the ground squirrel sequence that may aid GRP78 function under the near 0°C body temperatures of the hibernating state. Electrophoretic mobility shift and supershift assays showed that the activating transcription factor, ATF4, binds to the promoter region of the grp78 gene in ground squirrel brain and may be responsible for grp78 up-regulation during hibernation. Changes in grp78 gene and protein expression appear to aid stress tolerance in two highly oxygen-dependent organs that are critical to whole animal survival during hibernation.
Similar content being viewed by others
References
Wang LCH, Lee TF: Torpor and hibernation in mammals: metabolic, physiological and biochemical adaptations. In: MJ Fregley, CM Blatteis CM (eds). Handbook of Physiology: Environmental Physiology. Section 4, Oxford University Press, New York, 1: 507–532, 1996
Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM: Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 14: 193–205, 1994
Storey KB: Mammalian hibernation: transcriptional and translational controls. Adv Exp Med Biol 543: 21–38, 2003
Storey KB: Cold, ischemic organ preservation: lessons from natural systems. J Invest Med 52: 315–322, 2004
Storey KB, Storey JM: Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev 79: 207–233, 2004
Eddy SF, Storey KB: Up-regulation of fatty acid-binding proteins during hibernation in the little brown bat, Myotis lucifugus. Biochim Biophys Acta 1676: 63–70, 2004
Eddy SF, Morin P, Storey KB: Cloning and expression of PPARγ and PGC-1α from the hibernating ground squirrel, Spermophilus tridecemlineatus. Mol Cell Biochem 269: 175–182, 2005
Barja de Quiroga G: Brown fat thermogenesis and exercise: two examples of physiological oxidative stress? Free Radic Biol Med 13: 325–340, 1992
Carey HV, Frank CL, Seifert JP: Hibernation induces oxidative stress and activation of NF-kappaB in ground squirrel intestine. J Comp Physiol B 170: 551–559, 2000
Schroder M, Kaufman RJ: The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789, 2005
Kozutsumi Y, Segal M, Normington K, Gething M-J, Sambrook J: The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332: 462–464, 1988
Gething M-J: Role and regulation of the ER chaperone BiP. Cell Devel Biol 10: 465–472, 1999
Lee AS: The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26: 504–510, 2001
Chen Y, Matsushita M, Nairn AC, Damuni Z, Cai D, Frerichs KU, Hallenbeck JM: Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry 40: 11565–11570, 2001
Dignam JD, Lebovitz RM, Roeder RG: Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nuc Acids Res 11: 1475–1489, 1983
Roy B, Lee AS: The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nuc Acids Res 27: 1437–1443, 1999
Luo S, Baumeinster P, Yang S, Abcouwer SF, Lee AS: Induction of GRP78/BiP by translational block. Activation of the Grp78 promoter by ATF4 through an upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J Biol Chem 378: 37375–37385, 2003
Calvert ME, Digilio LC, Herr JC, Coonrod SA: Oolemmal proteomics — identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol 1: 27–37, 2003
Patil C, Walter P: Intracellular signalling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13: 349–355, 2001
Lee M, Choi I, Park K: Activation of stress signaling molecules in bat brain during arousal from hibernation. J Neurochem 82: 867–873, 2002
Eddy SF, McNally JD, Storey KB: Up-regulation of a thioredoxin peroxidase-like protein, proliferation associated gene, in hibernating bats. Arch Biochem Biophys 435: 101–111, 2005
Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME: Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C 133: 483–492, 2002
Osborne PG, Hashimoto M: Brain antioxidant levels in hamsters during hibernation, arousal and cenothermia. Behav Brain Res 168: 208–214, 2006
Hittel D, Storey KB: The translation state of differentially expressed mRNAs in the hibernating 13-lined ground squirrel (Spermophilus tridecemlineatus). Arch Biochem Biophys 401: 244–254, 2002
Boyer BB, Barnes BM: Molecular and metabolic aspects of mammalian hibernation. Bioscience 49: 713–724, 1999
Ramachandra KR, Changhui M, Baumeister P, Austin RC, Kaufman RJ, Lee AS: Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem 278: 20915–20924, 2003
Wooden SK, Lee AS: Comparison of the genomic organizations of the rat grp78 and hsc73 gene and their evolutionary implications. DNA Seq 3: 41–48, 1992
Pelham HRB: The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci 15: 483–486, 1990
Munro S, Pelham HRB: A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899–907, 1987
Hittel D, Storey KB: Differential expression of adipose and heart type fatty acid binding proteins in hibernating ground squirrels. Biochim Biophys Acta 1522: 238–243, 2001
Eddy SF, Storey KB: Up-regulation of fatty acid-binding proteins during hibernation in the little brown bat, Myotis lucifugus. Biochim Biophys Acta 1676: 63–70, 2004
Rutkowski DT, Kaufman RJ: (2004) A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28, 2001
Lee AS: The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35: 373–381, 2005
Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM: Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA 95: 14511–14516, 1998
Alexandre S, Nakaki T, Vanhamme L, Lee AS: A binding site for the cyclic adenosine 3′, 5′-monophosphate-response element binding protein as a regulatory element in the grp78 promoter. Mol Endocrinol 5: 1862–1878, 1991
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mamady, H., Storey, K.B. Up-regulation of the endoplasmic reticulum molecular chaperone GRP78 during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem 292, 89–98 (2006). https://doi.org/10.1007/s11010-006-9221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9221-8