Abstract
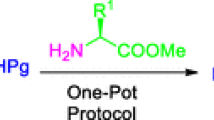
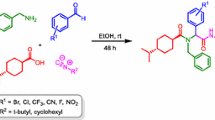
A simple and an efficient one-pot protocol for the synthesis of a new class of dithiocarbonate-tethered peptidomimetics and neo-glycosylated amino acids have been developed. This synthetic process involves in situ generation of a dithiocarbonate salt from the Nα-protected aminol substrate by reacting with CS2 followed by addition of bromo amino acid/sugar bromide to form the desired products. Amino acids with simple as well as bifunctional side chains were employed to obtain dithiocarbonate-tethered peptidomimetics in good yields. Further, the protocol was extended for the synthesis of N,N′-orthogonally protected dithiocarbonate-tethered peptidomimetics.
Graphical Abstract





Similar content being viewed by others
References
Alexander BH, Gertler SI, Oda TA, Bown RT, Ihndris RW, Beroza M (1960) New organic compounds for use in insect control. J Org Chem 25:626
Baker R, O’Mahony MJ, Swain CJ (1987) Total synthesis of (+)-milbemycin (β3). Perkin Trans I:1623
Baptise JL, Yemets S, Legay R, Lequeux T (2006) Synthesis of 2,3-trans disubstituted tetrahydrofurans through sequential xanthate radical addition-substitution reactions. J Org Chem 71:2352
Barton DHR, Combie SW (1975) A new method for the deoxygenation of secondary alcohols. Perkin Trans I:1574
Barton DHR, Jang DO, Jaszberenyi JC (1990) An improved radical chain procedure for the deoxygenation of secondary and primary alcohols using diphenylsilane as hydrogen atom donor and triethylborane-air as initiator. Tetrahedron Lett 31:4681
Barton DHR, Jang DO, Jaszberenyi JC (1991) Towards dideoxynucleosides: the silicon approach. Tetrahedron Lett 32:2569
Barton DHR, Chen M, Jaszberenyi JC, Rattingan B, Tang D (1994) Synthesis and application of chlorodithiocarbonates, thionothiocarbonates, and thionoselenocarbonates in radical chain reactions. Tetrahedron Lett 35:6457
Basavaprabhu Sureshbabu VV (2011) N,N′-carbonyldiimidazole-mediated dbu-catalyzed one-pot synthesis of urea-tethered glycosyl amino acids and glycoconjugates. Synlett 8:1160
Chabaud L, Landais Y, Renaud P (2005) Total synthesis of hyacinthacine a1 and 3-epi-hyacinthacine A1. Org Lett 7:2587
Chaturvedi D, Ray S (2006) An efficient, one-pot, triton-B catalyzed synthesis of O-alkyl-S-methyl dithiocarbonates. Monatshefte fur Chemie 137:1219
Chaturvedi D, Ray S (2007) A high yielding, one-pot synthesis of O,S-dialkyl dithiocarbonates from alcohols using Mitsunobu’s reagent. Tetrahedron Lett 48:149
Chugaev L (1899) Formation of olefins from alcohols without rearrangement through pyrolysis of the corresponding xanthates via cis-elimination. Ber 32:3332
Degani I, Fochi R, Regondi V (1979) The phase-transfer synthesis of unsymmetrical dialkyl sulfides via O,S-dialkyl dithiocarbonates. Synthesis 3:178
Degani I, Fochi R, Regondi V (1981) Rearrangement of O,S-dialkyl dithiocarbonates to S,S-dialkyl dithiocarbonates catalyzed by tricaprylmethylammonium chloride. Synthesis 2:149
Dehmel F, Weinbenner S, Julius H, Ciossek T, Maier T, Stengel T, Fettis K, Burkhsrdt C, Wieland H, Beckers T (2008) Trithiocarbonates as a novel class of HDAC inhibitors: SAR studies, isoenzyme selectivity, and pharmacological profiles. J Med Chem 13:3985
Deng S, Gangadharmath U, Chang CT (2006) Sonochemistry: a powerful way of enhancing the efficiency of carbohydrate synthesis. J Org Chem 71:5179
Goodman M, Zhang J (1997) Peptidomimetic building blocks for drug design. Chemtracts: Org Chem 10:629
Greene TW, Wuts PGM (1999) protective groups in organic synthesis, 3rd edn. Wiley Interscience, New York, p 484
Hartwig W (1983) Modern methods for the radical deoxygenation of alcohols. Tetrahedron 39:2609
Hemantha HP, Sureshbabu VV (2009) A simple approach for the synthesis of new classes of dithiocarbamate-linked peptidomimetics. Tetrahedron Lett 50:7062
Hemantha HP, Chennakrishnareddy G, Sureshbabu VV (2010) Synthesis of thioureido-linked peptidomimetics, glycosylated amino acids, and neoglycoconjugates using bis(benzotriazolyl)methanethione as thioacylating agent. Synlett 5:715
Isola M, Ciuffarin E, Sangromora L (1976) A convenient synthesis of optically active thiols. Synthesis 5:326
Kakaei S, Chen N, Xu J (2013) Expeditious synthesis of 1-substituted taurines with diverse functionalized side-chains. Tetrahedron 69:302
Katritzky RA, Witek MR, Garecia RV, Mohapatra PP, Rogers WJ, Cusido J, Abdel-Fattah AAA, Steel JP (2005) Benzotriazole-assisted thioacylation. J Org Chem 70:7866
Nace HR (1962) Chugaev reaction. Org React 12:57
Nagendra G, Madhu C, Vishwanatha TM, Sureshbabu VV (2012) An expedient route for the reduction of carboxylic acids to alcohols employing 1-propanephosphonic acid cyclic anhydride as acid activator. Tetrahedron Lett 53:5059
Narendra N, Lalithamba HS, Sureshbabu VV (2010) An efficient one-pot access to trithiocarbonate-tethered peptidomimetics. Tetrahedron Lett 51:6169
Okatawa M, Nakai T, Otsuji Y, Imoto E (1965) Photochemical behavior of o-ethyl s-benzyl xanthate as a model compound for a photosensitive resin. J Org Chem 30:2025
Olson GL, Bolin DR, Bonner MP, Bös M, Cook CM, Fry DC, Graves BJ, Hatada M, Hill DE, Kahn M, Madison VS, Rusiecki VK, Sarabu R, Sepinwall J, Vincent GP, Voss ME (1993) Concepts and progress in the development of peptidemimetics. J Med Chem 36:3039
Paladino J, Thurieau C, Morris AD, Kucharczyk N, Rouissi N, Regoli D, Fauchere JL (1993) Synthesis and in vitro activities of new tryptophan-modified and thiomethylene-containing pseudopeptide antagonists of the neurokinins. Int J Pept Protein Res 42:284
Patil BS, Vasanthakumar GR, Sureshbabu VV (2003) Isocyanates of N α-[(9-fluorenylmethyl)oxy]carbonyl amino acids: synthesis, isolation, characterization, and application to the efficient synthesis of urea peptidomimetics. J Org Chem 68:7274
Raichle K, Rossing L, Zorn H (1952) Sulphur containing organic compounds Ger Pat 840239
Ramanarao RV, Sureshbabu VV (2006) Synthesis of retro-inverso peptides employing isocyanates of N α-Fmoc-amino acids/peptide acids catalyzed by DMAP. Tetrahedron Lett 47:9139
Salvador LA, Elofsson M, Kihlberg J (1995) Preparation of building blocks for glycopeptide synthesis by glycosylation of Fmoc amino acids having unprotected carboxyl groups. Tetrahedron 51:5643
Salvatore NR, Sahab S, Jung KW (2001) Mild and efficient synthesis of thiocarbonates and thiocarbamates via a three-component coupling utilizing Cs2CO3 and TBAI. Tetrahedron Lett 42:2055
Sureshbabu VV, Patil BS, Ramanarao RV (2006) Preparation, isolation, and characterization of N α-fmoc-peptide isocyanates: solution synthesis of oligo-α-peptidyl ureas. J Org Chem 71:7697
Sureshbabu VV, Venkataramanarao R, Naik SA, Chennakrishnareddy G (2007) Synthesis of tetrazole analogues of amino acids using Fmoc chemistry: isolation of amino free tetrazoles and their incorporation into peptides. Tetrahedron Lett 48:7038
Sureshbabu VV, Sudarshan NS, Venkataramanarao R (2008a) Peptidyl carbamates: efficient synthesis using N-fmoc-β-aminoalkoxy carbonyl chlorides/active pentachlorophenyl carbonates as monomeric building blocks. Int J Pept Res Ther 14:149
Sureshbabu VV, Hemantha HP, Naik SA (2008b) Synthesis of 1,2,4-oxadiazole-linked orthogonally urethane-protected dipeptide mimetics. Tetrahedron Lett 49:5133
Sureshbabu VV, Naik SA, Hemantha HP, Narendra N, Das U, Row TNG (2009) N-urethane-protected amino alkyl isothiocyanates: synthesis, isolation, characterization, and application to the synthesis of thioureidopeptides. J Org Chem 74:5260
Tanaka K, Yamagichi N, Tanikaga R, Kaji A (1979) New methods for synthesis of α-β-unsaturated carboxylic esters from carbonyl compounds using monoanions of dithiocarbonates, and dianions of ethyl mercaptoacetate and ethyl 2-mercaptopropionate. Bull Chem Soc Japan. 52:3619
Thorn GD, Ludwig RA (1962) The dithiocarbamates and related compounds. Elsevier, Amsterdam
Yang D, Li B, Ng FF, Yan YL, Qu J, Wu YD (2001) Synthesis and characterization of chiral N-O turns induced by α-aminoxy acids. J Org Chem 66:7303
Yokoyama M, Imamoto T (1984) Organic reactions of carbon disulfide. Synthesis 10:797
Acknowledgments
We sincerely thankful to Council of Scientific and Industrial Research (CSIR) Grant No. 02(0149)/13/EMR-II Government of India, New Delhi for financial support.
Conflict of interest
Muniyappa Krishnamurthy, Basavaprabhu, Vommina V. Sureshbabu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Authors declare that there is no informed consent in the article. This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishnamurthy, M., Basavaprabhu & Sureshbabu, V.V. One-Pot Synthesis of New Class of Dithiocarbonate-Tethered Peptidomimetics. Int J Pept Res Ther 21, 195–203 (2015). https://doi.org/10.1007/s10989-014-9448-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-014-9448-2