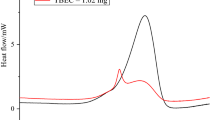
Abstract
Emulsion explosives (EE) are one of a typical industrial explosive that is widely used in engineering and mining applications due to its high detonation performance and ideal safety characteristics. However, since an EE is a multicomponent mixture with an oil-in-water structure, the presence of some substances or impurities can reduce the safety of the EE and cause accidents. We investigated the influence of ferrous ion on the thermal behavior and stability of EE during production and storage. The thermogravimetry–differential scanning calorimetry and water solubility method were selected for the assessment of thermal behavior and stability of the EE. According to kinetic parameters, the apparent activation energy (Ea) of the EE and the EE with ferrous ion additives was determined. The results indicated that ferrous ion could decrease the thermal decomposition temperature and Ea of EE. Moreover, the degree of crystallization of ammonium nitrate increases as storage time and ferrous ion content increases. It is of important significance to reduce the hazards of the EE and to improve both safety and stability during production and storage.










Similar content being viewed by others
References
Anshits AG, Anshits NN. Detonation velocity of emulsion explosive containing cenospheres. Combust Explos Shock Waves. 2005;41:591–8.
Deribas AA, Medvedev AE, Reshetnyak AY, Fomin VM. Detonation of emulsion explosive containing hollow microspheres. Dokl Phys. 2003;389(6):163–5.
Wang XG. Emulsion explosives. Beijing: Metallurgical Industry Press; 2008.
Mahadevan EG. Ammonium nitrate explosives for civil applications-slurries, emulsions and ammonium nitrate fuel oils. Weinheim: Wiley; 2013.
Medvedev AE, Fomin VM, Reshetnyak AY. Mechanism of detonation of emulsion explosive with microballoons. Shock Waves. 2008;18:107–15.
Xie XH, Wang L, Zhou HS. Enlightment of “May 20” explosion accident. Adv Mater Res. 2015;1082:391–4.
Xu S, Tan L, Liu JP, Chen X, Jiang W, Chen Y, Liu DB. Cause analysis of spontaneous combustion in an ammonium nitrate emulsion explosive. J Loss Prevent Process Ind. 2016;43:181–8.
Zhang KM, Ni OQ, Huang JD, Dai YM, Zhao HR. A facile and efficient method to investigate the effect of the nature of surfactant and continuous phase on the performance of emulsion explosive. J Mol Liq. 2018;249:203–10.
Irina M, Alexander YM. The engineering rheology of liquid explosives as highly concentrated emulsions. Chem Eng Res Des. 2013;91:204–10.
Mura E, Josset C, Loubar K, Bellettre J, Massoli P. Experimental study of the water in oil emulsions features by differential scanning calorimetry analysis. Appl Energy. 2012;97:834–40.
Califano V, Calabria R, Massoli P. Experimental evaluation of the effect of emulsion stability on micro-explosion phenomena for water-in-oil emulsions. Fuel. 2014;117:87–94.
Boyd J, Parkinson C, Sherman P. Factors affecting emulsion stability, and the HLB concept. J Colloid Interface Sci. 1972;41(2):359–70.
Mudeme S, Masalova I, Haldenwang R. Kinetics of emulsification and rheological properties of highly concentrated explosive emulsions. Chem Eng Process. 2010;49:468–75.
Sanatkaran N, Masalova I, Malkin AY. Effect of surfactant on interfacial film and stability of highly concentrated emulsions stabilized by various binary surfactant mixtures. Colloids Surf A Physicochem Eng Asp. 2014;461:85–91.
Nsenda NT, Irina M. Stabilization of highly concentrated emulsions with oversaturated dispersed phase: effect of surfactant/particle ratio. Chem Eng Res Des. 2015;102:216–33.
Wang S, Xu Z, Wang Q. Thermal decomposition mechanism of emulsion explosives with phosphatide. J Therm Anal Calorim. 2016;124:1053–62.
Sanatkaran N, Masalova I, Malkin AY. Effect of surfactant on interfacial film and stability of highly concentrated emulsions stabilized by various binary surfactant mixtures. Colloid Surf A Physicochem Eng Asp. 2014;461:85–91.
Ma Z, Zhou Y, Wang J. Influence of water content in emulsion explosives basic substance on their thermal decomposition and calculation of kinetic parameters. Chin J Explos Propellants. 2009;32:44–7.
Han Z, Sachdeva S, Papadaki MI, Mannan MS. Ammonium nitrate thermal decomposition with additives. J Loss Prevent Process Ind. 2015;35:307–15.
Xu ZX, Liu DB, Hu Y. Investigation of ammonium nitrate based emulsion ignition characteristic. J Loss Prevent Process Ind. 2013;26:994–1001.
Jones DE, Feng HT, Mintz KJ. Parameters affecting the thermal behaviour of emulsion explosives. Thermochim Acta. 1999;331:37–44.
Wang K, Xu S, Liu D. Research on the critical temperature of thermal decomposition for large cartridge emulsion explosives. J Loss Prevent Process Ind. 2015;38:199–203.
Gillard P, Longuet B. Investigation of heat transfer and heterogeneous reactions during the slow cook off of a composite propellant. J Loss Prevent Process Ind. 2013;26:1506–14.
Turcotte R, Goldthorp S, Badeen CM, Feng HT. Influence of physical characteristics and ingredients on the minimum burning pressure of ammonium nitrate emulsions. Propellants Explos Pyrotech. 2010;35:233–9.
Gunawan R, Zhang D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J Hazard Mater. 2009;165:751–8.
Xu ZX, Wang Q, Fu XQ. Thermal stability and mechanism of decomposition of emulsion explosives in the presence of pyrite. J Hazard Mater. 2015;300:702–10.
Sun JH, Sun Z, Wang Q, Ding H, Wang T, Jiang CS. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate. J Hazard Mater. 2005;127:204–10.
Xu ZX, Liu DB, Hu YT. Influence of iron ion on thermal behavior of ammonium nitrate and emulsion explosives. Cent Eur J Energ Mater. 2010;7:77–93.
Kaljuvee T, Edro E, Kuusik R. Influence of lime containing additives on the thermal behaviour of ammonium nitrate. J Therm Anal Calorim. 2008;92:215–21.
Yuan MH, Shu CM, Kossoy A. Kinetics and hazards of thermal decomposition of methyl ethyl ketone peroxide by DSC. Thermochim Acta. 2005;430:67–71.
Sankaranarayanan A, Mallick L, Kumbhakarna NR. A numerical and experimental study of the decomposition pathways of guanidinium nitrate. J Therm Anal Calorim. 2018;131:427–41.
Dalmazzone D, Hamed N, Christine D, Rousseau L. Application of high pressure DSC to the kinetics of formation of methane hydrate in water-in-oil emulsion. J Therm Anal Calorim. 2006;85(2):361–8.
Kousksou T, Jamil A, Gibout S, Zeraouli Y. Thermal analysis of phase change emulsion. J Therm Anal Calorim. 2009;96:841–52.
Skarlis SA, Berthout D, Dujardin C, Dujardinb C, Granger P. Combined experimental and kinetic modeling approaches of ammonium nitrate thermal decomposition. Thermochim Acta. 2014;584:58–66.
Jimmie CO, James LS, Evan-Rogers YuM. Ammonium nitrate: thermal stability and explosivity modifiers. Thermochim Acta. 2002;384:23–45.
Kajiyama K, Izato Y, Miyake A. Thermal characteristics of ammonium nitrate, carbon, and copper(II) oxide mixtures. J Thermal Anal Calorim. 2013;113:1475–80.
Djerdjev AM, Priyananda P, Gore J, Beattie JK, Neto C, Hawkett BS. The mechanism of the spontaneous detonation of ammonium nitrate in reactive grounds. J Environ Chem Eng. 2018;6:281–8.
Korošec RC, Kajič P, Bukovec P. Determination of water, ammonium nitrate and sodium nitrate content in ‘water-in-oil’ emulsions using TG and DSC. J Therm Anal Calorim. 2007;89:619–24.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;19:1702–6.
Wang XH, Li XJ, Yan HH. Research of thermal decomposition kinetic characteristic of emulsion explosive base containing Fe and Mn elements. J Therm Anal Calorim. 2008;91:545–50.
Warless EJ, Ducker WA. Weak influence of divalent ions on anionic surfactant surface-aggregation. Langmuir. 1997;13:1463–74.
Shinoda K, Hirai T. Ionic surfactants applicable in the presence of multivalent cations. Physicochemical properties. J Phys Chem. 1977;81:1842–5.
Mu JH, Li GZ, Jia XL, Liao LJ, Wen QZ. Rheological properties and microstructures of anionic micellar solutions in the presence of different inorganic salts. J Phys Chem B. 2002;106:11685–93.
Lucassen-Reynders EH. Anionic surfactants: physical chemistry of surfactant action. New York: Marcel Dekker; 1981.
Acknowledgements
The work was supported by the National Natural Science Foundation of China Coal Joint Fund (No. 51134012) and Anhui Provincial Educational Office Key Natural Science Fund (No. KJ2010A102).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, XH., Feng, YQ., Liu, SH. et al. Thermal behavior and stability of emulsion explosives in the presence of ferrous ion. J Therm Anal Calorim 139, 999–1006 (2020). https://doi.org/10.1007/s10973-019-08494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08494-0