Abstract
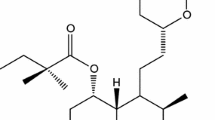
One of the major challenges for the development of drugs containing active pharmaceuticals ingredient (API) with low solubility is to add technologies to the development process in order to increase bioavailability while ensuring stability. Thus, the main objective of this work was to apply analytical methodologies in the characterization and stability of solid dispersions of atorvastatin calcium obtained by different techniques. The calorimetric curves showed different thermal profiles for the dispersions, presenting a greater interaction of the components, the SD-PEG sd. Moreover, the thermogravimetric curves showed that the PEG conferred greater stability to the dispersions. The FTIR/Pearson correlation and PXRD data revealed neither chemical interaction nor intense amorphization in the solid dispersions, respectively. Additionally, it was concluded the thermoanalytical techniques are useful, complementary, and elucidative in the characterization of the physical–chemical nature of the solid dispersions obtained by lyophilization and spray drying, providing reliable results regarding the thermal behavior and the solubility of the samples.













Similar content being viewed by others
References
Yun F, Kang A, Shan J, Zhao X, Bi X, Li J. Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement. Int J Pharm. 2000. https://doi.org/10.1016/j.ijpharm.2014.02.040.
Choi H, Lee H, Lee MK, Lee J. Polymer-directed crystallization of atorvastatin. J Pharma Sci. 2012. https://doi.org/10.1002/jps.23206.
Hu L, Gu D, Hu Q, Shi Y, Gao N. Investigation of solid dispersion of atorvastatin calcium in polyethylene glycol 6000 and polyvinylpyrrolidone. Trop J Pharma Sci Res. 2014;13:835–42.
de Waard H, Hinrichs WL, Visser MR, Bologna C, Frijlink HW. Unexpected differences in dissolution behavior of tablets prepared from solid dispersions with a surfactant physically mixed or incorporated. Int J Pharma. 2008;349:66–73.
Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharma Biopharm. 2013;85:799–813.
Jung HJ, Ahn HI, Park JY, Ho MJ, Lee DR, Cho HR, Park JS, Choi YS, Kang MJ. Improved oral absorption of tacrolimus by a solid dispersion withhypromellose and sodium lauryl sulfate. Int J Biol Macromol. 2016;83:282–7.
Bikiaris DN. Solid dispersions, Part I: recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2011;8:1501–19.
Srinarong P, de Waard H, Frijlink HW, Hinrichs WLJ. Improved dissolution behavior of lipophilic drugs by solid dispersions: the production process as starting point for formulation considerations. Expert Opin Drug Deliv. 2011. https://doi.org/10.1517/17425247.2011.598147.
Dan Smithey PG, Taylor L. Amorphous solid dispersions: an enabling formulation technology for oral delivery of poorly water-soluble drugs. AAPS Newsmagazine. 2013;16:11–4.
Adibkiaa K, Barzegar-Jalalia M, Maheri-Esfanjanib H, Ghanbarzadeh S, Shokri J, Sabzevari A, Javadzadeh J. Physicochemical characterization of naproxen solid dispersions prepared via spray drying technology. Powder Technol. 2013;246:448–55.
Choudhary A, Rana AC, Geeta Aggarwal GA, Kumar V, Zakir F. Development and characterization of an atorvastatin solid dispersion formulation using skimmed milk for improved oral bioavailability. Acta Pharma Sin B. 2012;2:421–8.
Chung NO, Lee MK, Lee J. Mechanism of freeze-drying drug nanosuspensions. Int J Pharma. 2012;437:42–50.
Van Drooge DJ, Braeckmans K, Hinrichs WLJ, Remaut K, De Smedt SC, Frijlink HW. Characterization of the mode of incorporation of lipophilic compounds in solid dispersions at the nanoscale using fluorescence resonance energy transfer (FRET). Macrom R Com. 2014;27:1149–55.
Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420:1–10.
Kim MS, Jin SJ, Kim JS, Park HJ, Song HS, Neubert RH, Hwang SJ. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 2018;69:454–65.
Jahangiri A, Barzegar-Jalali M, Garjani A, Javadzadeh Y, Hamishehkara H, Afroozian A, Adibkia K. Pharmacological and histological examination of atorvastatin-PVP K30 solid dispersions. Powder Technol. 2015;286:538–45.
Palanisamy M, James A, Khanam J. Atorvastatin e cyclodextrin systems: physiochemical and biopharmaceutical evaluation. J Drug Deliv Sci Technol. 2016;31:41–52.
Qi B, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study—assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7.
Afonso Moura E, Correia LP, Pinto MF, Procópio JVV, Souza FS, Macedo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Calorim. 2010. https://doi.org/10.1007/s10973-009-0473-x.
Boer TM, Procopio JVV, Nascimento TG, Macedo RO. Correlation of thermal analysis and pyrolysis coupled to GC MS in the characterization of tacrolimus. J Pharm Biomed Anal (Print). 2013;73:18–23.
Procópio JVV, Souza VG, Costa RA, Correia LP, Souza FS, Macêdo RO. Application of thermal analysis and pyrolysis coupled to GC/MS in the qualification of simvastatin pharmaceutical raw material. J Therm Anal Calorim. 2011;106:665–70.
Soares MFLR, Soares-Sobrinho JL, Silva KER, Alves LDS, Lopes PQ, Correia LP, Souza FS, Macedo RO, Neto PJR. Thermal characterization of antimicrobial drug ornidazole and its compatibility in a solid pharmaceutical product. J Therm Anal Calorim. 2011;104:307–13.
Costa SPM, Silva KER, Medeiros GCR, Rolim LA, Oliveira JF, Lima MCA, Galdino SL, Pitta IR, Neto PJR. Thermal behavior and compatibility analysis of the new chemical entity LPSF/FZ4. Thermochem Acta. 2013;562:29–34.
Leite RS, Macedo RO, Batista CCN, Baltazar LOB, Lima Neto SA, Souza SS. Evaluation of thermal stability and parameters of dissolution of nifedipine crystals. J Therm Anal Calorim. 2013. https://doi.org/10.1007/s10973-012-2605-y.
Medeiros AFD, Santos AFO, Souza FS, Procopio JVV, Pinto MF, Macedo RO. Thermal Stability of paracetamol and its pre-formulates obtained by spray drying. J Therm Anal Calorim. 2007;88(2):377–82.
Ramos FJL, Silva KMA, Brandão DO, Chaves JV, Santos JAB, Andrade FHD, Batista RSA, Lins TB, Sousa DP, Medeiros ACD, Conceição MM, Macêdo RO, Souza FS. Investigation of the thermal behavior of inclusion complexes with antifungal activity. 2018; https://doi.org/10.1007/s10973-018-7040-2.
Da Silva RMF, Medeiros FPM, Nascimento TG, Macedo RO, Neto PJR. Thermal characterization of indinavir sulfate using TG, DSC and DSC-photovisual. J Therm Anal Calorim. 2009;95:965–8.
Daniel JSP, Veronez IP, Rodrigues LL, Trevisan MG, Garcia JS. Risperidone—solid-state characterization and pharmaceutical compatibility using thermal and non-thermal techniques. Therm Acta. 2013;568:148–55.
Cavallari C, Tarterini F, Fini A. Thermal characterization of some polymorph solvates of theanti-inflammatory/anti-cancer sulindac. Thermochim Acta. 2016;633:129–39.
Chienga N. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Dias SBT, Nascimento TG, Santos AFO, Viana IMMN, Almeida RM, Junior ID, Macedo RO, Araújo-Júnior JX. Polymorphic characterization and compatibility study of clozapine: implications on its stability and some biopharmaceutics properties. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-014-4142-3.
Correia LP, Procópio JVV, Santana CP, Pinto MF, Moura EA, Santos AFO, Macedo RO. Herbal medicine physical quality evaluation by thermal analysis using adapted Ozawa method. J Therm Anal Calorim. 2015. https://doi.org/10.1007/s10973-015-4638-5.
Fernandes FHA, Santana CP, Santos RL, Correia LP, Conceição MM, Macêdo RO, Medeiros ACD. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J Therm Anal Calorim. 2012. https://doi.org/10.1007/s10973-012-2807-3.
Correia LP, Santana CP, Medeiros ACD, Macêdo RO. Sideroxylon obtusifolium herbal medicine characterization using pyrolysis GC/MS, SEM and different thermoanalytical techniques. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-015-4986-1.
Brandão DO, Guimarães GP, Santos RL, Ramos FJL, Silva KMA, Souza FS, Macêdo RO. Model analytical development for physical, chemical, and biological characterization of Momordica charantia vegetable drug. J Anal Method Chem. 2016. https://doi.org/10.1155/2016/7528297.
Correia LP, Santana CP, Silva KMA, Ramos FJL, Lima RSC, Souza FS, Medeiros ACD, Macêdo RO. Physical and chemical characteristics of Maytenus rigida in different particle sizes using SEM/EDS, TG/DTA and pyrolysis GC–MS. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-016-5999-0.
Cartaxo-Furtado NÃO, Brandão DO, Júnior FJLR, Silva KMA, Macêdo RO. Investigation of thermal and kinetic behavior of the Stryphnodendron adstringens dry extract with antimicrobial activity. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08047-5.
Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the curves study). Am J Cardiol. 2011;81:582–7.
Lima IPB, Lima NGPB, Barros DMC, Oliveira TS, Barbosa EG, Gomes APB, Ferrari M, do Nascimento TG, Aragão CFS. Compatibility study of tretinoin with several pharmaceutical excipients by thermal and non-thermal techniques. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-014-4295-0.
Devore JL. Probability and statistics for engineering and the sciences. 4th ed. Belmont: Duxbury Press; 1995.
Hupp AM, Marshall LJ, Campbell DI, Smith RW, McGuffin VL. Chemometric analysis of diesel fuel for forensic and environmental applications. Anal Chim Acta. 2008;606:159–71.
Paudel A, Worku ZA, Meeus J, Guns S, Mooter GV. Manufacturing of solid dispersions of poorly water-soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453:253–84.
Mahlin D, Ponnambalam S, Höckerfelt MH, Bergström CAS. Toward in silico prediction of glass-forming ability from molecular structure alone. A screening tool in early drug development. Mol Pharm. 2011;8:498–506.
Baird JA, Santiago-Quinonez D, Rinaldi C, Taylor LS. Role of viscosity in influencing the glass-forming ability of organic molecules from the undercooled melt state. Pharm Res. 2012. https://doi.org/10.1007/s11095-011-0540-4.
Sonje VM, Kumar L, Puri V, Kohli G, Kaushal AM, Bansal AK. Effect of counterions on the properties of amorphous atorvastatin salts. Eur J Pharm Sci. 2011;44:462–70.
Medeiros AFD, Santos AFO, Souza FS, Procópio JVV, Pinto MF, Macêdo RO. Thermal stability of paracetamol and its Pre-formulates obtained by spray drying. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-006-8006.
Shukat R, Bourgaux C, Relkin P. Crystallisation behaviour of palm oil nanoemulsions carrying vitamin E DSC and synchrotron X-ray scattering studies. J Therm Anal Calorim. 2012. https://doi.org/10.1007/s10973-011-1846-5.
Herbrink M, Schellens JHM, Beijnen JH, Nuijen B. Improving the solubility of nilotinib through novel spray-dried solid dispersions. Int J Pharm. 2017. https://doi.org/10.1016/j.ijpharm.2017.07.010.
Zhang HX, Wang JX, Zhang ZB, Le Y, Shen ZG, Chen JF. Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm. 2009;374:106–13.
Shayanfar A, Jouybar A. Drug–drug coamorphous systems: characterization and physicochemical properties of coamorphous atorvastatin with caverdilol and glibenclamide. J Pharm Innov. 2013;8:218–28.
Ha ES, Baek IH, Cho W, Hwang SJ, Kim MS. Preparation and evaluation of solid dispersion of atorvastatin calcium with Soluplus® by spray drying technique. Chem Pharm Bull. 2014;62:545–51.
Lavor EP, Navarro MVM, Freire FD, Aragão CFS, Raffin FN, Barbosa EG, Moura TFAL. Application of thermal analysis to the study of antituberculosis drugs-excipient compatibility. J Therm Anal Calorim. 2014;115:2303–9.
Silva EP, Pereira MAV, Lima IPB, Lima NGPB, Barbosa EG, Aragão CFS, Gomes APB. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-015-5077-z.
Dametto PR, Dametto AC, Polese L, Ribeiro CA, Chorilli M, Freitas O. Development and physicochemical characterization of solid dispersions containing praziquantel for the treatment of schistosomiasis. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-016-5759-1.
Panghal D, Nagpal M, Thakur GS, Arora S. Dissolution improvement of atorvastatin calcium using modified locust bean gum by the solid dispersion technique. Sci Pharm. 2014;82:177–91.
Reginald-Opara JN, Attama A, Ofokansi K, Umeyor C, Kenechukwu F. Molecular interaction between glimepiride and Soluplus1-PEG 4000 hybrid based solid dispersions: characterisation and anti-diabetic studies. Int J Pharm. 2015;2015(496):741–50.
Souza CMP, Santos JAB, Nascimento AL, Júnior JVC, Júnior FJLR, Neto SAL, Souza FS, Macêdo RO. Thermal analysis study of solid dispersions hydrochlorothiazide. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-017-6091-0.
Sharma B, Saini V, Sharma A. Preparation, characterization and in-vitro evaluation of atorvastatin calcium solid dispersions with various hydrophilic polymers and its FDT formulation. Curr Pharm Res. 2012;2:620–30.
Hu L, Gu D, Hu Q, Shi Y, Gao N. Investigation of solid dispersion of atorvastatin calcium in polyethylene glycol 6000 and polyvinylpyrrolidone. Trop J Pharm Res. 2014;13:835–42.
Soulairol I, Tarlier N, Bataille B, Cacciaguerra T, Sharkawi T. Spray-dried solid dispersions of nifedipine and vinylcaprolactam/vinylacetate/PEG6000 for compacted oral formulations. Int J Pharm. 2015;481:140–7.
Fong SIK, Ibisogl YA, Bauer-brandl A. Solubility enhancement of BCS Class II drug by solid phospholipid dispersions: spray drying versus freeze-drying. Int J Pharm. 2015;496:382–91.
Maggio RM, Vignaduzzo SE, Kaufman TS. Practical and regulatory considerations for stability-indicating methods for the assay of bulk drugs and drug formulations. Trends Anal Chem. 2013;49:57–70.
Chaudhari BG, Patel NM, Shah PB. Stability indicating RP-HPLC method for simultaneous determination of atorvastatin and amlodipine from their combination drug products. Chem Pharm Bull. 2007;55:241–6.
Kadav AA, Vora DN. Stability indicating UPLC method for simultaneous determination of atorvastatin, fenofibrate and their degradation products in tablets. J Pharm Biomed Anal. 2008;48:120–6.
Acknowledgements
The authors would like to thank the “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)” and the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)” for supporting this work. Additionally, the authors would like to thank Felipe Ramos for support to translate this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, K.M.A., de Lima Ramos Júnior, F.J., Júnior, J.V.C. et al. Characterization of solid dispersions of a powerful statin using thermoanalytical techniques. J Therm Anal Calorim 138, 3701–3714 (2019). https://doi.org/10.1007/s10973-019-08450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08450-y