Abstract
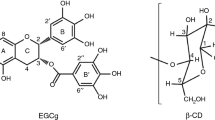
The formation mechanism of inclusion complex between (-)-epigallocatechin gallate (EGCg), which is the main catechin in tea leaves, or (-)-gallocatechin gallate (GCg), which is an isomer of EGCg, and β-cyclodextrin (βCD) was studied by isothermal titration microcalorimetry (ITC), NMR and molecular modeling calculation (MMC). ITC measurements revealed that EGCg or GCg interacted with βCD at 1:1 molar ratio driven by enthalpy. Comparing the values of binding constant (7.2 × 103 1/M for EGCg with βCD and 3.9 × 104 1/M for GCg with βCD), it was suggested that GCg interacted with βCD more strongly rather than EGCg. In their ROESY NMR spectra, the cross-peaks were observed between the protons of A, B and B′ ring of EGCg and the protons in the cavity of βCD, and between the protons of B and B′ ring of GCg and the protons in the cavity of βCD. MMC in water showed that EGCg had one kind and GCg had two kinds of most stable conformation (GCg(E) and GCg(A)) in energy. In the stable conformation of EGCg, B ring was coordinated equatorially to C ring, and B′ ring was axially coordinated to C ring. On the other hand, in GCg (E), the coordination of B ring and B′ ring to C ring was both equatorial, and in GCg (A), the coordination of B ring and B′ ring was both axial. MMC also revealed B or B′ ring of GCg(A) was easy to be included in the cavity of βCD more deeply than each ring of EGCg or GCg(E) since the steric hindrance of B ring or B′ ring was small. Therefore, it was found that the difference of B ring’s configuration of gallate-type catechin greatly affected the formation of inclusion complex with βCD.










Similar content being viewed by others
References
Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075–87.
Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem Biol Interact. 2008;171:89–95.
Grzesik M, Naparlo K, Bartosz G, Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chem. 2018;241:480–92.
Braicu C, Ladomery MR, Chedea VS, Irimie A, Berindan-Neagoe I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013;141:3282–9.
Szejtli J, Szente L. Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur J Pharm Biopharm. 2005;61:115–25.
Ikeda H, Fukushige Y, Matsubara T, Inenaga M, Kawahara M, Yukawa M, Fujisawa M, Yukawa E, Aki H. Improving water solubility of nateglinide by complexation of β-cyclodextrin. J Therm Anal Calorim. 2016;123:1847–50.
Tonnesen HH, Masson M, Loftsson T. Studies of curcumin and curcuminoids. XXXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244:127–35.
Guo Z, Wu F, Singh V, Guo T, Ren X, Yin X, Shao Q, York P, Patterson LH, Zhang J. Host-guest kinetic interactions between HP-β-cyclodextrin and drugs for prediction of bitter taste masking. J Pharm Biomed Anal. 2017;140:232–8.
Junior OV, Dantas JH, Barao CE, Zanoelo EF, Cardozo-Filho L, de Moraes FF. Formation of inclusion compounds of (+)catechin with β-cyclodextrin in different media: spectral, thermal and antioxidant properties. J Supercrit Fluids. 2017;121:10–8.
Ho S, Thoo YY, Young DJ, Siow LF. Inclusion complexation of catechin by β-cyclodextins: characterization and storage stability. LWT Food Sci Technol. 2017;86:555–65.
Acknowledgements
The computations were performed using Research Center for Computational Science, Okazaki, Japan and Research Institute for Information Technology of Kyushu University, Fukuoka, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeda, H., Ohata, T., Yukawa, M. et al. Difference in formation mechanism of inclusion complex between configuration isomers of gallate-type catechin and β-cyclodextrin. J Therm Anal Calorim 135, 2789–2795 (2019). https://doi.org/10.1007/s10973-018-7680-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7680-2