Abstract
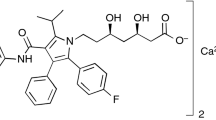
The aim of this study was to characterize atorvastatin and evaluate interactions between atorvastatin and various excipients by DSC and FTIR, using Pearson’s correlation as a tool to corroborate possible interactions that it was not possible to evidence in visual analyses. The DSC curves were obtained using a Shimadzu calorimeter, Model DSC-60, in the aluminum crucible under heating rate of 20 °C min−1 at a temperature of 25–400 °C. The spectra of the samples were obtained on a FTIR–ATR model IR prestige-21 Shimadzu spectrophotometer at a wavelength of 700–4000 cm−1 on average of 20 scans. The theoretical spectrum was obtained using an ad hoc algorithm. From the analysis of DSC and evaluation of Pearson’s correlation, it observed physical interactions with excipients: starch glycolate, pre-gelatinized starch, croscarmellose, sodium lauryl sulfate, magnesium stearate and mannitol. There is no interaction with lactose. Then, the Pearson’s correlation was so important tool to evaluate possible interactions between IPAs and excipients, using FTIR data to corroborate DSC results.






Similar content being viewed by others
References
Oliveira MA, Yoshida MI, Belinelo VJ, Valotto RS. Degradation kinetics of atorvastatin under stress conditions and chemical analysis by HPLC. Molecules. 2013;18:1447–56.
United State Pharmacopeial, The United States Pharmacopeial Convention. United States Pharmacopeia. 34th ed. Rockville (MD, USA): The United States Pharmacopeial Convention, 2010.
Zerbini APN. Desenvolvimento e avaliação de minicomprimidos contendo atorvastatina cálcica. Universidade de São Paulo, 2010.
Mendonça CMS, Lima IPB, Aragão CFS, Gomes APB. Thermal compatibility between hydroquinone and retinoic acid in pharmaceutical formulations. J Therm Anal Calorim. 2013. doi:10.1007//s10973-013-2941-6.
Freire FD, Aragão CF, Lima TFA, Raffim FN. Compatibility study between chlorpropamide and excipients in their physical mixtures. J Therm Anal Calorim. 2009;97:355–7.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–55.
Bertol CD, Cruz AP, Stulzer HK, Murakami FS, Silva MAS. Thermal decomposition kinetics and compatibility studies of primaquine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2010;102:187–92.
Pinto MF, Moura EA, Souza FS, Macêdo RO. Thermal compatibility studies of nitroimidazoles and excipients. J Therm Anal Calorim. 2010;102:323–9.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipientes. J Therm Anal Calorim. 2010;99:631–7.
Peres-Filho MJ, Gaeti MPN, Oliveira SR, Marreto RN, Lima EM. Thermoanalytical investigation of olanzapine compatibility with excipients used in solid oral dosage forms. J Therm Anal Calorim. 2011;104:255–60.
Song VM, Kumar L, Puri V, Kohli G, Kaushal AM, Bansal A. Effect of counterions on the properties of amorphous atorvastatin salts. Eur J Pharm Sci. 2011;44:462–70.
Zhang HX, Wang JX, Zhang ZB, LE Y, Shen ZG, Chen JF. Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm. 2009;374:106–13.
Jin YS, Ulrich J. New Crystalline Solvates of Atorvastatin Calcium. Chem Eng Technol. 2010;33(5):839–44.
Lima IPB, Lima NGPB, Barros DMCB, Oliveira TSO, Barbosa EG, Gomes APB, Ferrari M, Nascimento TGN, Aragão CFS. Compatibility study of tretinoin with several pharmaceutical excipients by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120:733–47. doi:10.1007/s10973-014-4295-0.
An SG, Sohn YT. Crystal forms of atorvastatin. Arch Pharm Res. 2009;32(6):933–6. doi:10.1007/s12272-009-1616-0933.
Carvalho KP, Rocha TC, Leles MIG. Characterization of atorvastatin by TG and DSC. Braz J Therm Anal. 2012;1:79–83.
Kim MS, Jin SJ, Kim JS, Park HJ, Song HS, Neubert RHH, Hwang SJ. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 2008;69(2):454–65.
Yoshida MI, Oliveira MA, Lacerda CD, Bonella AF, Valotto RS. Caracterização Térmica da Atorvastatina e Estudos de Compatibilidade de Formulações Farmacêuticas. Br J Thermal Anal. 2012;1:73–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, E.P., Pereira, M.A.V., de Barros Lima, I.P. et al. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim 123, 933–939 (2016). https://doi.org/10.1007/s10973-015-5077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5077-z