Abstract
Thermogravimetry (TG) and differential scanning calorimetry (DSC) are used in pharmaceutical studies for characterization of drugs, purity, compatibility of formulations, identification of polymorphism, evaluation of stability, and thermal decomposition of drugs and pharmaceutical formulations. Hydroquinone (HQ) and products containing HQ have been widely used as depigmentation agents for lightening the skin. Retinoids are compounds that have the basic core structure of vitamin A and its oxidized metabolites, or synthetic compounds that share similar mechanisms of action as naturally occurring retinoids. Depigmentants and excipients were analyzed by TG and DSC. The dynamic thermogravimetric curves were obtained on a SHIMADZU thermobalance, model DTG-60, using an alumina crucible, at the heating rate of 10 °C min−1, in the temperature range of 25–900 °C, under an atmosphere of nitrogen at 50 mL min−1. The sample's mass was 10 ± 0.05 mg. The DSC curves were obtained using Shimadzu calorimeter, model DSC-60, using aluminum crucible, at the heating rate of 10 °C min−1, in the temperature range of 25–400 °C. The thermogravimetric and calorimetric curves were analyzed using TASYS software SHIMADZU. In this study were found the interaction between retinoic acid (RA) and the following excipients: cetyl alcohol(CA), cetostearyl alcohol (CTA), glycerin(GLY), and dipropylene glycol (DPG), and that between HQ and the excipient, DPG. Therefore, additional studies are necessary to evaluate final formulations. Thermal analysis is an effective and reliable technique that can be used in the control of raw materials and pharmaceutical products, and for evaluating their employment potential in the development and characterization of products.
Similar content being viewed by others
Introduction
Thermal analysis comprises a group of techniques that allows evaluating the physical properties of a drug and/or its reaction products, while the drug is subjected to a controlled temperature programming. The main use of thermal analysis focuses on the study of transition temperature (melting and boiling, besides the presence of solvates, liquid crystals, glass transitions, and isomerization), heat of transition, purity, compatibility, and decomposition kinetics of polymorphic transition. The advantages of thermal analysis in checking the effect of the heating treatment on the materials rely on great precision, accuracy, and sensitivity, for being used as a permanent record [1].
The evaluation of drug stability and their possible physical and chemical interactions involves the incompatibility study, as the interactions can affect the chemical nature, the stability, and bioavailability of drugs and, consequently, their therapeutic efficacy and safety. Differential Scanning Calorimetry (DSC) has been proposed as a rapid method to examine the possibility of physicochemical interactions, between drug(s) and drugs and excipient(s), predicting eutectic behavior of drugs and excipients for the preparation of a phase diagram. Differential thermal analysis (DTA) has been proposed for predicting polymorphic forms and examining thermal behavior kinetic analysis through thermogravimetry/derivative thermogravimetry curves (TG/DTG), since it is often necessary to predict degradation rates at marketing temperatures on accelerated processes studied at elevated temperatures [2].
Thermogravimetry/derivative thermogravimetry (TG/DTG) and DSC techniques are widely applied and used in pharmaceutical analyses [3]. These techniques are used in studies of characterization, purity, compatibility of formulations, identification of polymorphism, evaluation og stability of drugs, and thermal decomposition of drugs' and pharmaceutical formulations [4]. Thermal analysis also allows for the determination of important information about the physical properties of materials and about kinetic parameters (activation energies, frequency factor, and reaction order) which can be measured by thermoanalytic methods according to progress of reactions [5, 6].
Melanin is the key pigment in human skin responsible for scavenging harmful UV radiations and protecting the skin from harmful effects of toxic drugs and chemicals. It is also the determinant of skin color and tone. Melanin is produced by melanocytes in the basal layer of epidermis and then transferred to the adjacent keratinocytes through melanosomes [7].
People with different skin colors have the same number of melanocytes, but differing in the frequency of melanin gene expression and the packaging of this melanin into melanosomes. The number, size, and density of melanosomes transferred to the keratinocytes or the nature and degradation rate of the pigment are some of the variations that are shown to be present in different people. There are, however, certain disorders such as melasma, post-inflammatory hyperpigmentation, freckles, which are characterized by abnormal accumulation of melanin at specific areas in the skin. These disorders can cause a significant negative impact on a person’s health and quality of life, and thus, there is a great demand for the development of hypopigmenting agents [7].
Traditionally, there have been a number of pigmentation control agents that have been used and are marketed as different formulations. These agents act on different targets in melanin synthesis cycle, such as inhibition of melanocyte stimulation by UV radiation, irritation or free radicals (most of the antioxidants and anti-inflammatory agents), inhibition of tyrosinase glycosylation (n-acetyl glucosamine and tunicamycin), inhibition of melanin synthesis of enzyme tyrosinases (hydroquinone, resorcinols, kojic acid, arbutin, and ascorbic acid), or inhibition of melanosome transfer to keratinocytes (niacinamide, protease inhibitors) [7].
The most effective lightening agent of all these, which is also considered to be the gold standard in treating pigmentation disorders in the United States, is hydroquinone (HQ). It has been reported to improve hyperpigmentation in 14–70 % of the patients at concentrations of 2 % and is commonly used at a concentration of 4 % by dermatologists. At concentration above, this it is known to cause cytotoxicity to melanocytes as well as other side effects such as irritation, allergic dermatitis, permanent hypermelanosis, or amelanosis. Owing to these side effects, there has been a constant search for the development of a new and effective skin-lightening agent. Formulation optimization, skin irritation, and efficacy characterization form the basic considerations in the development of a novel skin-lightening agent [7].
Hydroquinone has been shown to induce reversible hypopigmentation in humans and animals. This is most likely caused by melanocyte destruction, decreased melanosome formation, altered melanosome structure, increased melanosome degradation, and destruction of membranous organelles [8].
Human skin color stems from the outermost layer of the skin, the epidermis, where the pigment-producing cells melanocytes are located to produce melanin. Upon exposure of the skin to UV radiation, melanogenesis is enhanced by the activation of the key enzyme of melanogenesis, tyrosinase [9].
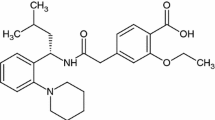
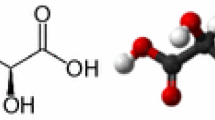
Hydroquinone and products containing HQ have been widely used as de-pigmentation agents to lighten skin. Its mechanism of action is based on the inhibition of melanin formation [10]. HQ, a hydroxyphenolic chemical compound (Fig. 1a), inhibits the conversion of DOPA to melanin by inhibiting the tyrosinase enzyme. It may also function by interfering with the formation or degradation of melanosomes and by inhibiting the synthesis of DNA and RNA within melanocytes. Its chemical resemblance with certain melanin precursors (tyrosine and dihydroxyphenylalanine) explains its ability to be metabolized in melanocytes as well as its selective action on melaninogenesis [11].
Topical HQ in 2–4 % concentrations, alone or in combination with tretinoin 0.05–0.1 %, has been successfully used for years in treating melasma. The problem with the use of HQ is that it can irritate the skin, causing dermatitis upon contact, particularly in higher concentrations of 4 % or greater, or when combined with tretinoin. HQ is also an unstable and easily oxidized substance in cosmetic formulations [12].
Vitamin A, its derivatives, and beta-carotene (pro vitamin A) have been popular among additives used in cosmetics for many years. The primary benefit of vitamin A and its derivatives in cosmetic products has been their ability to normalize keratinization. The popular derivatives of vitamin A that are used in cosmetics include vitamin A alcohol (retinol), vitamin A esters (retinyl palmitate or retinyl acetate), vitamin A aldehyde (retinal), and tretinoin (retinoic acid, RA). This family of compounds called retinoids has shown receptor-specific effects on the skin, resulting in decreased roughness and decreased facial wrinkling. Tretinoin (all trans-retinoic acid) is considered the most bioactive retinoid for modulating skin function [13].
Retinoids, such as vitamin A acid and RA or tretinoin, were first used in combination with HQ to enhance the penetration of HQ, but were later recognized to be having their own effect on the pigment. The RA (Fig. 1b) has the ability to disperse keratinocyte pigment granules, interfere with pigment transfer, and accelerate epidermal turnover and, therefore, pigment loss. In addition, there is also evidence that it can inhibit the induction of tyrosinase, DOPA chrome conversion factor, and melanogenesis [14].
All-trans-retinoic acid inhibits melanin synthesis through down-regulation of tyrosinase and tyrosinase-related protein 1 expression in melanocytes. All-trans-retinoic acid could also act as a selective melanocytotoxin in relatively high doses. In addition, RA increases keratinocyte turnover and augments melanin loss from the epidermis. The inhibition of glutathione S-transferase by RA explains its synergistic depigmenting action with other melanocytotoxins such as HQ. Topical retinoids could irritate the skin causing redness, dryness, swelling, desquamation, and subjective feelings such as itching, stinging, and burning sensation [13, 14].
There are no previous studies found in the literature on HQ and RA interactions, or on the Active Pharmaceutical Ingredients (APIs) with excipients, and the use of these formulations is common by pharmaceutical industry. Therefore, this study aims to develop a thermoanalytic method to analyze the compatibility between the HQ and RA with its excipients using TG and DSC.
Experimental
Samples and binary mixtures
Samples used in this study were retinoic acid (RA)(Galena, 1007034701), hydroquinone (HQ) (Vetec, 1000617), and excipients: disodium ethylenediaminetetraacetate (EDTA Na), Hydroxyethylmethyl Cellulose (HPMC), decyl oleate (DCO), methylparaben (MTP), proprylparaben (PPB), cetostearyl alcohol (CTA), cetyl alcohol (CA), Imidazolydinylurea (IMD), glycerin (GLY) and dipropylene glycol (DPG) were all obtained from Henrifarma and Galena. The binary mixtures were prepared at 1:1(w/w).
Differential Scanning Calorimetry (DSC)
DSC curves of APIs and binary mixtures have been recorded using a Shimadzu calorimeter model DSC-60. The operating conditions were nitrogen (flow rate: 50 mL min−1) and heating rate: 10 °C min−1 up to 400 °C. Aluminum sample pans with 2.0 mg of samples were used. The calibration was done using the melting points of the standards indium (mp 156.6 °C). The samples curves were analyzed using TASYS software provided by Shimadzu.
Thermogravimetry (TG)
The dynamic thermogravimetric curves of APIs as well as those of the binary mixtures were recorded using Shimadzu thermobalance, model DTG-60. About 6–10 mg of samples were placed in alumina crucible, and subjected to nitrogen flow (50 mL min−1), at heating rates of 10 °C min−1 in the temperature range of 25–900 °C. The calibration was done with calcium oxalate monohydrate. The samples curves were analyzed using TASYS software by Shimadzu.
Results and discussion
Characterization of retinoic acid, hydroquinone, and binary mixture between retinoic acid and hydroquinone by DSC
Figure 2 shows that the DSC curve of HQ has two endothermic events; the first corresponds to the melting process where the onset temperature was 176 °C and enthalpy of 358 J g−1 and followed by the decomposition event with onset temperature of 186 °C and enthalpy of 559 J g−1. The same figure presents RA with endothermic processes: the first probably corresponds to a change of state with onset temperature of 155 °C and enthalpy of 10 J g−1, while the second corresponds to melting event with onset temperature 187 °C and enthalpy of 149 J g−1. Studies on the thermal behavior of HQ and RA interactions are not found in the literature.
The binary mixture between RA and HQ (Fig. 2) presented three endothermic events. We hypothesized that there was, in the first event, a process of change in physical state (perhaps polymorphic transition) that can be caused by RA, where the onset temperature was 151 °C with enthalpy of 4 J g−1. In the second event occurred a process of melting with onset temperature in 166 °C and enthalpy of 69 J g−1, corresponding to the melting point of the HQ and the last event at temperature of 172 °C and enthalpy of 62 J g−1 that is the fusion of RA.
The melting events of the substances studied appear at 10 and 14 °C below melting endothermic peaks of the HQ and RA, respectively. Another relevant factor can be the difference in the enthalpies, with a remarkable lowering in fusion enthalpy of the substances of 289 and 80 J g−1 to HQ and RA, respectively. The thermal behavior of mixture can be attributed also to a decrease in melting peak due to the 1:1 mixture, which represents an impurity for the pure compounds.
Characterization of retinoic acid, hydroquinone, and binary mixture between retinoic acid and hydroquinone by TG
The TG curve of RA (Fig. 3) shows only three stage of decomposition in the temperature range of 185–609 °C (Table 1). HQ showed two stages of decomposition in the range of 110–253 °C (Table 2).
The binary mixtures of HQ and RA showed four stages of decomposition. The TG curves presented behavior of two APIs. In the first stage loss at 131–235 °C, the binary mixture presented thermal profile of HQ until 50 % of loss. At about 235 °C started in the binary mixtures the behavior of RA. Therefore, we understand that there were no interactions but well-defined behaviors of APIs.
Binary mixture between retinoic acid and excipients
Thermogravimetric studies between RA and different excipients showed different thermal behaviors with regard to the number of mass loss stages (Table 1). The TG curves of binary mixtures (Fig. 4) show until five stages of decomposition, and also a smaller onset of decomposition with MTP, PPB, CA, CTA, EDTA, and DPG (Table 1).
The TG curves with MTP show four stages of the thermal decomposition between 120 and 248 °C with mass loss of 99.6 %. The binary mixture between RA and MTP (Fig. 4) showed four stages of decomposition at 120–604 °C. This TG curve shows thermal decomposition at about 62 % in the first stage characteristic of MTP, and the second stage about 222 °C presented profile thermal behavior of the RA, it did not exhibit instability (Table 1).
The TG curves of PPB show that the substance was thermally stable up to 126 °C and decomposition occurred in single stage, with mass loss of 100 %. The TG curves of RA and PPB (Fig. 4) show four stages of decomposition between 136 and 770 °C, and despite thermal behaviors of the two substances, it exhibits stability.
The TG curves of the CA show single stage of the thermal decomposition between 138 and 473 °C with mass loss of 100 %. The TG curves of RA and CA (Fig. 4) show four stages of decomposition between 145 and 546 °C. In this case there is interaction between RA and CA, but the stability is improved because the start decomposition temperature increased.
Studies of interaction performed by Neto et al. [15], about compatibility between Prednicarbate e excipient showed that MTP, propylparaben and CA present only one step of decomposition, different from our findings [15].
The TG curves of the CTA show the first stage of the thermal decomposition between 130 and 326 °C with mass loss of 100 %. Binary mixture between RA and CTA (Fig. 4) showed that the decomposition occurred in four stages. The decomposition of RA–CTA mixture presented the same behavior as the RA–CA mixture (Table 1), because the initial decomposition temperature was increased (155 °C) and therefore improved the stability.
Thus, we believe that the CA and CTA act as thermoprotective excipients. Then, the temperature of the displacement increases to higher decomposition temperatures of these binary mixtures (RA–CA and RA–CTA).
Thermal behavior of the HPMC presented three stages of the thermal decomposition between 36 and 900 °C with a mass loss of 97 %. Binary mixture between RA and HPMC (Fig. 4) showed that the decomposition occurred in four stages. The first stage of mass loss begins at 187 °C, and we believe that the results did not exhibit interactions (Fig. 4).
Cavalheiro and coworkers [16] obtained the same results in their TG curves of HPMC with verapamil. Their study showed a mass loss of approximately 2 % between room temperature until 180 °C, while decomposition event had appeared in a single stage but in multiple events, showing that both components decomposed individually. This result did not exhibit important interaction with the active substance [16].
Thermal behavior of the GLY presented two stages of the thermal decomposition between 32 and 261 °C with mass loss of 100 %, while RA–GLY mixture presented four stages which can be seen in the Table 1. The first event of decomposition had occurred at 33 °C and corresponds to the humidity absorption. The second mass loss was about 129 °C, before the isloation of GLY (144 °C) and RA (185 °C), that led us to the suspicion of the probable interaction due to manipulation in a mortar and pestle, as is usually practiced in pharmacies.
Thermal behavior of the DCO presented two stages of the thermal decomposition between 167 and 323 °C with mass loss of 98 %. Binary mixture between RA and DCO showed that the decomposition occurred in three steps, and the beginning was at 165 °C close to the the RA isolated. Therefore, we believed that there were no interactions.
Thermal behavior of the EDTA Na presented five stages of the thermal decomposition in the temperature ranges of 78–193, 193–278, 278–360, 360–411, and 411–900 °C with mass losses of 10, 16, 12, 18, and 21 %, respectively. RA–EDTA had five stages as shown in Table 1. This result did not show interactions because the binary mixture presented thermal behaviors of the EDTA Na and RA individually.
Thermal behavior of the DPG presented one stage of the thermal decomposition between 33 and 184 °C with mass loss of 99 %. The binary mixture between RA and DPG (Fig. 4) showed five stages of decomposition in temperature range at 41–611 °C (Table 1). We believe that there is interactions in the binary mixture because appears two mass loss in the temperature range of the 41–185 °C is not characteristic of the DPG alone (one stage). The third mass loss of decomposition of RA–DPG corresponding the RA and this temperature there is not changes. But, the first and second stages, changes in the steps of DPG occured, and we hypothesize that this indicates interactions.
Binary mixture between hidroquinona and excipients
Binary mixture curves between HQ and MTP and HQ and PPB (Fig. 5) presented two stages between temperature ranges of 111–897 and 123–600 °C, respectively. It can be observed that for mixtures of HQ–PPB and HQ–MTP, the onset of decomposition of the two compounds alone did not alter the breakdown in either of them (Table 2). This result did not show interactions.
Binary mixture curves between HQ and CA (Fig. 5) presented two stages between 111 and 600 °C, with mass loss of 100 %. The binary mixture curve between HQ and CTA presented only one step between 120 and 252 °C, with mass loss of 100 %. This result did not show interactions (Table 2).
TG curves between HQ and HPMC presented four stages between 32 and 891 °C, with a total mass loss of 97 %, showing that both compounds decomposed individually. This result did not present important interaction with the active substance.
The TG curves for HQ–GLY and HQ–DCO show that three stages of thermal decomposition occur between 32–600 and 123–812 °C, respectively. The binary mixtures did not show any interaction (Table 2). The binary mixtures behave thermally as individual samples.
TG curve of HQ–EDTA Na show seven stages of mass loss, but it did not show interactions, and we have seen the behaviors of individual samples on the mixture.TG curve of HQ–DPG presents two stages of decomposition in the temperature range of 37–230 °C, presenting different thermogravimetric profiles from those observed for DPG. The binary mixture HQ–DPG showed a possible interaction that takes the behavior of HQ.
Conclusions
The DSC and TG serve the useful means for quick assessment of chemical interaction of drugs with excipients, as well as evaluattion of stability of pharmaceuticals and binary mixtures. In this study were found interactions between RA and the following excipients: CA, CTA, GLY, and DPG, and between HQ and the following excipient: DPG. Thermal analysis is an effective and reliable technique that can be used both in the control of raw materials, pharmaceuticals products, and employment potential in the development and characterization of products.
References
De Oliveira GGG, Ferraz HG, Severino P, Souto BE. Analysis of phase transition and dehydration processes of nevirapine. J Therm Anal Calorim. 2012;108:53–7.
Lavor EP, Freire FD, Aragão CFS, Raffin FN, Moura TFL. Application of thermal analysis to the study of anti-tuberculosis drug compatibility. Part 1. J Therm Anal Calorim. 2012;108:207–12.
Almeida MM, Lima CRRC, Quenca-Guillen JS, Filho EM, Mercuri LP, Santoro MIRM. Stability evaluation of tocopheryl acetate and ascorbyl tetraisopalmitate in isolation and incorporated in cosmetic formulations using thermal analysis. Brazilian J Pharm Sci. 2010;46:129–34.
Oliveira M, Yoshida M, Gomes E. Análise térmica aplicada a fármacos e formulações farmacêuticas na indústria farmacêutica. Quim Nova. 2011;34(7):1224–30.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behavior, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
Alves R, Reis TVS, Silva LCC, Storpírtis S, Mercuri LP, Matos JR. Thermal behavior and decomposition kinetics of rifampicin polymorphs under isothermal and non-isothermal conditions. Brazilian J Pharm Sci. 2010;46:343–51.
Jain P, Sonti S, Garruto J, Mehta R, Banga AK. Formulation optimization, skin irritation, and efficacy characterization of a novel skin-lightening agent. J Cosmet Dermatol. 2012;11:101–10.
Westerhof W, Kooyers TJ. Hydroquinone and its analogues in dermatology—a potential health risk. J Cosmet Dermatol. 2005;4:55–9.
Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cosmet Sci. 2011;33:210–21.
Lin C-H, Sheu J-Y, Wu H-L, Huang Y-L. Determination of hydroquinone in cosmetic emulsion using microdialysis sampling coupled with high-performance liquid chromatography. J Pharm Biomed Anal. 2005;38:414–9.
Katsambas AD, Stratigos AJ. Depigmenting and bleaching agents: coping with hyperpigmentation. Clin Dermatol. 2001;19:483–8.
Gao X-H, Zhang L, Wei H, Chen H-D. Efficacy and safety of innovative cosmeceuticals. Clin Dermatol. 2008;26:367–74.
Lupo MP. Antioxidants and vitamins in cosmetics. Clin Dermatol Y. 2001;19:467–73.
Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol. 2006;55:1048–65.
Neto HS, Novak CS, Matos JR. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97:367–74.
Nunes RS, Semaan FS, Riga AT, Cavalheiro ETG. Thermal behavior of verapamil hydrochloride and its association with excipients. J Therm Anal Calorim. 2009;97:349–53.
Acknowledgements
The authors are grateful for the support of the Brazilian Government's Research Gand the last rants (CNPQ, FAPERN, and PROPESQ/UFRN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Mendonça, C.M.S., de Barros Lima, I.P., Aragão, C.F.S. et al. Thermal compatibility between hydroquinone and retinoic acid in pharmaceutical formulations. J Therm Anal Calorim 115, 2277–2285 (2014). https://doi.org/10.1007/s10973-013-2941-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2941-6