Abstract
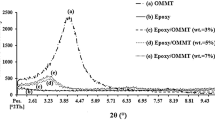
Epoxy–clay nanocomposites have been prepared with an organically modified montmorillonite. The epoxy network was based on diglycidyl ether of bisphenol A (DGEBA) cured with diaminodiphenylmethane (DDM). The stoichiometry DGEBA–DDM was varied, the molar ratio of amine hydrogen/epoxy groups, r, ranged from 0.85 to 1.15. The influence of stoichiometry on curing and properties of the nanocomposites was studied using differential scanning calorimetry, dynamic mechanical thermal analysis and X-ray diffraction. All nanocomposites had intercalated clay structures. The clays accelerated the curing reaction whose rate was also increased when increasing r. The heat of reaction, −ΔH (J/g epoxy), increased as r increased, reaching a constant value for r ≥ 1. In the presence of clays −ΔH was lower than in the neat DGEBA–DDM. The glass transition temperature (T g) of the neat epoxy thermosets reached a maximum at r = 1; however, the nanocomposites showed the T g maximum at 0.9 < r < 1. The presence of clay lowered the T g for r > 0.94 and raised T g for r ≤ 0.85. The elastic modulus of neat epoxy thermosets reached a maximum in the rubber state and a minimum in the glassy state at r = 1. The nanocomposites showed similar behavior, but the maximum and the minimum values of the elastic modulus were reached at stoichiometry r < 1. The comparison of the properties of neat epoxy with those of the nanocomposites varying the stoichiometry indicates that the clay itself induces stoichiometric changes in the system.











Similar content being viewed by others
References
LeBaron PC, Wang Z, Pinnavaia TJ. Polymer-layered silicate nanocomposites: an overview. Appl Clay Sci. 1999;15:11–29.
Pavlidou S, Papaspyrides CD. A review on polymer-layered silicate nanocomposites. Prog Polym Sci. 2008;33:1119–98.
Paul DR, Robeson LM. Polymer nanotechnology: nanocomposites. Polymer. 2008;49:3187–204.
Betega de Paiva LB, Morales AR, Valenzuela-Díaz FR. Organoclays: properties, preparation and applications. Appl Clay Sci. 2008;42:8–24.
Becker O, Simon GP. Epoxy layered silicate nanocomposites. Adv Polym Sci. 2005;179:29–82.
Qiu J, Wang S. Enhancing polymer performance through graphene sheets. J Appl Polym Sci. 2011;119:3670–4.
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS. Graphene-based polymer nanocomposites. Polymer. 2011;52:5–25.
Nuhiji B, Attard D, Thorogood G, Hanley T, Magniez K, Fox B. The effect of alternate heating rates during cure on the structure-property relationships of epoxy/MMT clay nanocomposites. Compos Sci Technol. 2011;71:1761–8.
Prolongo MG, Martinez-Casado FJ, Masegosa RM, Salom C. Curing and dynamic mechanical thermal properties of epoxy/clay nanocomposites. J Nanosci Nanotechnol. 2010;10:2870–9.
Lan T, Kaviratna PD, Pinnavaia TJ. Epoxy self-polymerization in smectite clays. J Phys Chem Solids. 1996;57:1005–10.
Wang Z, Pinnavaia TJ. Hybrid organic-inorganic nanocomposites: exfoliation of magadiite nanolayers in an elastomeric epoxy polymer. Chem Mater. 1998;10:1820–6.
Messersmith PB, Giannelis EP. Synthesis and characterization of layered silicate–epoxy nanocomposites. Chem Mater. 1994;6:1719–25.
Triantafillidis CS, LeBaron PC, Pinnavaia TJ. Thermoset epoxy–clay nanocomposites: the dual role of α,ω-diamines as clay surface modifiers and polymer curing agents. J Solid State Chem. 2002;167:354–62.
Park J, Jana SC. Effect of plasticization of epoxy networks by organic modifier on exfoliation of nanoclay. Macromolecules. 2003;36:8391–7.
Brown JM, Curliss D, Vaia RA. Thermoset-layered silicate nanocomposites. Quaternary ammonium montmorillonite with primary diamine cured epoxies. Chem Mater. 2000;12:3376–84.
Lan T, Pinnavaia TJ. Clay-reinforced epoxy nanocomposites. Chem Mater. 1994;6:2216–9.
Hussain F, Chen J, Hojjati M. Epoxy–silicate nanocomposites: cure monitoring and characterization. Mater Sci Eng A. 2007;467:445–6.
Nigam V, Setua DK, Mathur GN, Kar KK. Epoxy–montmorillonite clay nanocomposites: synthesis and characterization. J Appl Polym Sci. 2004;93:2201–10.
Garea S, Iovu H, Stoleriu S, Voicu G. Synthesis and characterization of new nanocomposites based on epoxy resins and organophilic clays. Polym Int. 2007;56:1106–14.
Gupta VB, Drzal LT, Lee CY. The temperature-dependence of some mechanical properties of a cured epoxy resin system. Polym Eng Sci. 1985;25:812–23.
Palmese GR, McCullough RL. Effect of epoxy–amine stoichiometry on cured resin material properties. J Appl Polym Sci. 1992;46:1863–73.
Meyer F, Sanz G, Eceiza A, Mondragon I, Mijovic J. The effect of stoichiometry and thermal history during cure on structure and properties of epoxy networks. Polymer. 1995;36:1407–14.
Munz M, Sturm H, Stark W. Mechanical gradient interphase by interdiffusion antiplasticisation effect-study and epoxy/thermoplastic system. Polymer. 2005;46:9097–112.
Sánchez-Cabezudo M, Prolongo MG, Salom C, Masegosa RM. Cure kinetics of epoxy resin and thermoplastic polymer. J Therm Anal Calorim. 2006;86:699–705.
Román F, Montserrat S, Hutchinson JM. On the effect of montmorillonite in the curing reaction of epoxy nanocomposites. J Therm Anal Calorim. 2007;1:113–8.
Zvetkov VL, Krastev RK, Samichkov VI. Rate equations in the study of the DSC kinetics of epoxy–amine reactions in an excess of epoxy. Thermochim Acta. 2008;478:17–27.
Sherman LC, Zeigler RC, Verghese NE, Marks MJ. Structure–property relationships of controlled epoxy networks with quantified levels of excess epoxy etherification. J Polymer. 2008;49:1164–72.
Macan J, Brnardic I, Ivancovic M, Mencer HJ. DSC study of cure kinetics of DGEBA-based epoxy resin with poly(oxypropilene) diamine. J Therm Anal Calorim. 2005;81:369–73.
Sánchez-Cabezudo M, Masegosa RM, Salom C, Prolongo MG. Correlations between the morphology and the thermo-mechanical properties in poly(vinyl acetate)/epoxy thermosets. J Therm Anal Calorim. 2010;102:1025–33.
Gude MR, Prolongo SG, Ureña A. Effect of the epoxy/amine stoichiometry on the properties of carbon nanotube/epoxy composites. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2056-x.
Acknowledgements
Financial support by Ministerio de Educación y Ciencia of Spain (MAT 2009-11083) and by Universidad Politécnica de Madrid-Investigation Groups Support is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García del Cid, M.A., Prolongo, M.G., Salom, C. et al. The effect of stoichiometry on curing and properties of epoxy–clay nanocomposites. J Therm Anal Calorim 108, 741–749 (2012). https://doi.org/10.1007/s10973-012-2215-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2215-8