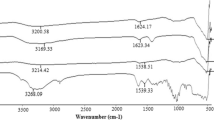
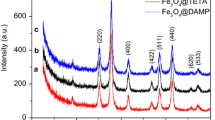
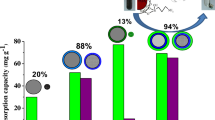
Abstract
The surface of magnetite nanoparticles was coated with functional polysiloxane layers using reaction of hydrolytic copolycondensation of tetraethoxysilane and 3-aminopropyltriethoxysilane (or N-[3-trimethoxysilylpropyl]ethylendiamine), and also that of tetraethoxysilane, 3-aminopropyltriethoxysilane and methyltriethoxysilane (or n-propyltriethoxysilane). It was shown that these functionalized magnetically controllable particles (about 60–150 nm in size as aggregates), as opposed to magnetite, adsorb urease well from aqueous solutions (up to 1 g/g), and that the level of residual activity of adsorbed layers is up to 84 % in the case of a bifunctional sample. It was established that the activity of immobilized urease is normally gradually reduced during storage of the samples, but in the case of ethylenediamine functional group is not decreased for 45 days. The synthesized samples are promising for use as magnetically directed biocatalysts.






Similar content being viewed by others
References
Eltekova NA, Eltekov AY (2000) Sieve effect at the adsorption of albumin and gamma globulin. Russ J Phys Chem 74:1075–1079 (in Russian)
Van der Veen M, Cohen Stuart M, Norde W (2007) Spreading of proteins and its effect on adsorption and desorption kinetics. Colloids Surf B Biointerfaces 54:136–142
Hofs B, Brzozowska A, de Keizer A et al (2008) Reduction of protein adsorption to a solid surface by a coating composed of polymeric micelles with a glass-like core. J Colloid Interface Sci 325:309–315
Tsapikouni ThS, Missirlis YF (2008) Protein-material interactions: from micro-to-nano scale. Mat Sci Eng B 152:2–7
Reichelt S, Eichhorn KJ, Aulich D et al (2009) Functionalization of solid surfaces with hyperbranched polyesters to control protein adsorption. Colloids Surf B Biointerfaces 69:169–177
Braun S, Rappoport S, Zusman R, Avnir D (1990) Ottolenghi M. Biochemically active sol–gel glasses. The trapping of enzymes. Mater Lett 10:1–8
Brinker CJ, Sheerer GW (1990) Sol–gel science. The physics and chemistry of sol–gel processing. Academic Press, San Diego
Kandimalla VB, Tripathi VS, Ju H (2006) Immobilization of biomolecules in sol–gels: biological and analytical applications. Crit Rev Anal Chem 36:73–106
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. Wiley, New York
Avnir D, Coradin T, Lev O, Livage J (2006) Recent bio-applications of sol–gel materials. J Mater Chem 16:1013–1030
Coradin T, Boissiere M, Livage J (2006) Sol–gel chemistry in medicinal science. Curr Med Chem 13:99–108
Pogorilyi RP, Goncharyk VP, Yurchenko GR et al (2005) Immobilization of urease on functionalized polisyloxane xerogels and hydrogels obtained by sol–gel method. Quest Chem Chem Technol 6:103–106 (in Ukraininan)
Takahashi H, Li B, Sasaki T, Miyazaki C, Kajino T, Inagaki S (2001) Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater 44–45:755–762
Pimentel MCB, Leao ABF, Melo EHM, Ledingham WM, Lima-Filho JL, Sivewright M, Kennedy JF (2007) Artificial cells. Blood Substit Biotechnol 35:221–235
Bayramoglu G, Arıca MY (2008) Preparation of poly(glycidylmethacrylate–methylmethacrylate) magnetic beads: application in lipase immobilization. J Mol Catal B Enzym 55:76–83
Ma Z, Guan Y, Liu H (2006) Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. J Magn Magn Mat 301:469–477
Melnik IV, Zub YuL, Alonso B, Abramov NV, Gorbik PP (2012) Creating of a functional polysiloxane layer on the surface of magnetic nanoparticles using sol–gel method. Glass Phys Chem 38:96–104
Melnyk IV, Zub YL (2012) Preparation and characterisation of magnetic nanoparticles with bifunctional surface layer ≡ Si(CH2)3NH2/≡ SiCH3 (or ≡ SiC3H7–n). Microporous Mesoporous Mater 154:196–199
Brunauer JS, Emmet PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Lurie YY (1984) Analytical chemistry of industrial wastewater. Chemistry, Moscow (in Russian)
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinium. J Am Chem Soc 40:1361–1403
Finn L, Slinyakova I (1975) The structure and thermal decomposition of the organopolysiloxane xerogels shown by IR spectroscopy. Colloid J 37:723–729 (in Russian)
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Academic Press, London
Giles CH, MacEwan TH, Nakhwa SN et al (1960) A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 10:3973–3993
Bosker WTE, Iakovlev PA, Norde W, Cohen Stuart M (2005) BSA adsorption on bimodal PEO brushes. J Colloid Interface Sci 286:496–503
Pogorilyi RP, Goncharyk VP, Kozhara LI, Zub YL (2009) The influence on the activity of the adsorbed urease of structural and adsorption characteristics of polisyloxane matrices containing surface layer with 3-aminopropyl groups. Surface 1(16):35–45 (in Ukrainian)
Pogorilyi RP, Goncharyk VP, Kozhara LI, Zub YuL (2008) Covalent immobilization of urease on polysiloxane matrices containing 3-aminopropyl and 3-mercaptopropyl groups. Appl Biochem Microbiol 44:621–625 (in Russian)
Pogorilyi RP, Honcharyk VP, Melnyk IV, Zub YuL (2008) In: Innocenzi P, Zub Yu, Kessler V (eds) Sol–gel methods for materials processing, XII. Springer, Dordrecht
Acknowledgments
R. P. P., I. V. M. and Yu. L. Z. would like to thank the State Target Scientific and Technical Program “Nanotechnologies and Nanomaterials” (project 6.22.5.42) and TCPFR “Fundamental Problems of Nanostructural Systems, Nanomaterials, and Nanotechnologies” (project no. 57/12-H) of NAS of Ukraine. V. G. K. and G. A. S. express their gratitude to the Swedish Research Council for support of the project “Molecular Precursors and Molecular Models of Nanoporous Materials” and to the EU FP7 program for support of the EuRARE project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Pogorilyi, R.P., Melnyk, I.V., Zub, Y.L. et al. Urease adsorption and activity on magnetite nanoparticles functionalized with monofunctional and bifunctional surface layers. J Sol-Gel Sci Technol 68, 447–454 (2013). https://doi.org/10.1007/s10971-013-2991-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-2991-z