Abstract
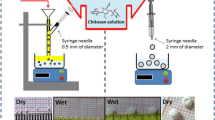
A typical type of natural zeolite(Z) modified with chitosan was applied to remove U(VI) from aqueous solution. Batch experiments were performed to investigate effects of pH, initial U(VI) concentration and contact time. Results show that chitosan-modified zeolite (CZ) exhibited enhanced adsorption capacity for U(VI) and rapider adsorption kinetics than Z. The optimal pH ranges from 5 to 7. U(VI) adsorption data of Z and CZ could be described by pseudo-first order kinetic model and Langmuir isotherm model. Adsorption mechanism of U(VI) on Z and CZ was studied by means of SEM, XRD and FT-IR.









Similar content being viewed by others
References
Abed AM, Sadaqah R, Al KM (2008) Uranium and potentially toxic metals during the mining, beneficiation, and processing of phosphorite and their effects on ground water in Jordan. Mine Water Environ 27(3):171–182
Kim J, Tsouris C, Mayes RT et al (2013) Recovery of uranium from seawater: a review of current status and future research needs. Sep Sci Technol 48(3):367–387
Ogawa T, Minato K, Okamoto Y, Nishihara K (2007) Nuclear energy and waste management—pyroprocess for system symbiosis. J Nucl Mater 360(1):12–15
Basu A, Brown ST, Christensen JN et al (2015) Isotopic and geochemical tracers for U(VI) reduction and U mobility at an in situ recovery U mine. Environ Sci Technol 49(10):5939–5947
Luo MB, Bo-Ping LI, Zhi Y et al (2008) Preconcentration techniques for uranium(VI) prior to analytical determination—an overview. Uranium Geol 24(1):57–62
Wang X, Fan Q, Yu S et al (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 281:448–455
Panja S, Mohapatra PK, Tripathi SC et al (2012) A highly efficient solvent system containing TODGA in room temperature ionic liquids for actinide extraction. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2012.06.015
Kim GN, Bin SD, Park HM et al (2011) The development of precipitation-filtering technology for uranium electrokinetic leachate. Sep Purif Technol 79(2):144–150
Tavakoli H, Sepehrian H, Semnani F, Samadfam M (2013) Recovery of uranium from UCF liquid waste by anion exchange resin CG-400: breakthrough curves, elution behavior and modeling studies. Ann Nucl Energy 54:149–153
Korichi S, Bensmaili A (2009) Sorption of uranium (VI) on homoionic sodium smectite experimental study and surface complexation modeling. J Hazard Mater 169(1–3):780–793
Crane RA, Scott T (2014) The removal of uranium onto carbon-supported nanoscale zero-valent iron particles. J Nanopart Res 16(12):2813
Li Y, Li L, Yu J (2017) Applications of zeolites in sustainable chemistry. Chem 3(6):928–949
Zhou L, Boyd CE (2014) Total ammonia nitrogen removal from aqueous solutions by the natural zeolite, mordenite: a laboratory test and experimental study. Aquaculture 432:252–257
Markou G, Vandamme D, Muylaert K (2014) Using natural zeolite for ammonia sorption from wastewater and as nitrogen releaser for the cultivation of Arthrospira platensis. Bioresour Technol 155:373–378
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156(1):11–24
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280(2):309–314
Liang Z, Shi W, Zhao Z et al (2017) The retained templates as “helpers” for the spherical meso-silica in adsorption of heavy metals and impacts of solution chemistry. J Colloid Interface Sci 496:382–390
Ezzeddine Z, Batonneau-Gener I, Pouilloux Y et al (2018) Synthetic nax zeolite as a very efficient heavy metals sorbent in batch and dynamic conditions. Colloids Interfaces 2(2):22
Tangkawanit S, Rangsriwatananon K (2004) Exchange diffusion of Cu2+, Ni2+, Pb2+ and Zn2+ into analcime synthesized from perlite. Microporous Mesoporous Mater 75(3):273–279
Querol X, Moreno N, Umaa JC et al (2002) Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol 50(1):413–423
Vocciante M, De Folly D’Auris A, Finocchi A et al (2018) Adsorption of ammonium on clinoptilolite in presence of competing cations: investigation on groundwater remediation. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.07.025
Baek W, Ha S, Hong S et al (2018) Cation exchange of cesium and cation selectivity of natural zeolites: chabazite, stilbite, and heulandite. Microporous Mesoporous Mater 264:159–166
Hernández-Montoya V, Pérez-Cruz MA, Mendoza-Castillo DI et al (2013) Competitive adsorption of dyes and heavy metals on zeolitic structures. J Environ Manag 116:213–221
Dimirkou A, Doula MK (2008) Use of clinoptilolite and an Fe-overexchanged clinoptilolite in Zn2+ and Mn2+ removal from drinking water. Desalination 224(1–3):280–292
Dyer A (2007) Natural zeolites by G. V. Tsitsishvili, T. G. Andronikashvili, G. R. Kirov and L. D. Filizova. Ellis Horwood, Chichester 1991. No. of pages: 297. Price: £69.00 (hardback). ISBN 0 13 612037 7. Geol J. https://doi.org/10.1002/gj.3350290217
Sprynskyy M, Buszewski B, Terzyk AP, Namieśnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304(1):21–28
Huynh J, Palacio R, Safizadeh F et al (2017) Adsorption of uranium over NH2-functionalized ordered silica in aqueous solutions. ACS Appl Mater Interfaces 9(18):15672–15684
Zhou L, Zou H, Wang Y et al (2016) Adsorption of uranium(VI) from aqueous solution using magnetic carboxymethyl chitosan nano-particles functionalized with ethylenediamine. J Radioanal Nucl Chem 308(3):935–946
Parab H, Joshi S, Shenoy N et al (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresour Technol 96(11):1241–1248
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Modifified chitosan and calcium alginate biopolymer sorbents for removal of nickel(II) through adsorption. Carbohydr Polym 72:261–271
Erdem B, Özcan A, Özcan AS (2010) Adsorption and solid phase extraction of 8-hydroxyquinoline from aqueous solutions by using natural bentonite. Appl Surf Sci 256(17):5422–5427
Zhang WM, Liu HY, Fan XR, Zhuo Z, Guo YD (2019) Removal of uranium from aqueous solution by a permeable reactive barrier loaded with hydroxyapatite-coated quartz sand: implication for groundwater remediation. Chem Erde. https://doi.org/10.1016/j.chemer.2019.125545
Khan TA, Dahiya S, Khan EA (2017) Removal of direct red 81 from aqueous solution by adsorption onto magnesium oxide-coated kaolinite: isotherm, dynamics and thermodynamic studies. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.12432
Wu FC, Wu PH, Tseng RL, Juang RS (2014) Use of refuse-derived fuel waste for the adsorption of 4-chlorophenol and dyes from aqueous solution: equilibrium and kinetics. J Taiwan Inst Chem Eng 45(5):2628–2639
Gao Y, Shao Z, Xiao Z (2015) U(VI) sorption on illite: effect of pH, ionic strength, humic acid and temperature. J Radioanal Nucl Chem 303(1):867–876
Liu X, Xu Y, Jin R et al (2014) Facile synthesis of hierarchical Fe4(P2O7)3 for removal of U(VI). J Mol Liq. https://doi.org/10.1016/j.molliq.2014.10.021
Wang J, Chen Z, Shao D et al (2017) Adsorption of U(VI) on bentonite in simulation environmental conditions. J. Mol, Liq, p 242
Han H, Cheng C, Hu S et al (2017) Facile synthesis of gelatin modified attapulgite for the uptake of uranium from aqueous solution. J Mol Liq 234:172–178
Liu T, Wu K, Zeng L (2012) Removal of phosphorus by a composite metal oxide adsorbent derived from manganese ore tailings. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2012.01.019
Azari A, Gharibi H, Kakavandi B et al (2016) Magnetic adsorption separation process: an alternative method of mercury extracting from aqueous solution using modified chitosan coated Fe3O4 nanocomposites. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.4990
Guzel P, Aydın YA, Deveci Aksoy N (2016) Removal of chromate from wastewater using amine-based-surfactant-modified clinoptilolite. Int J Environ Sci Technol 13(5):1277–1288
Bakharev T (2005) Resistance of geopolymer materials to acid attack. Cem Concr Res 35(4):658–670
Golubeva OY, Mokeev MV (2016) Study of the influence of extra-framework cations and organic templates on zeolite crystallization in SiO2–Al2O3–Na2O–K2O (R2O, RO) systems. Glas Phys Chem 42(6):566–575
Bok TO, Knyazeva EE, Ivanova II (2018) Specific features of formation of crystalline silicoaluminophosphates in grains based on kaolin and phosphoric acid. Russ J Appl Chem 91(6):948–958
Sureshkumar MK, Das D, Mallia MB, Gupta PC (2010) Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J Hazard Mater 184(1–3):65–72
Nasirmahaleh HH, Safi SZ, Sazegar MR et al (2016) Adsorption of amoxicillin on surfactant modified zeolites and their antibacterial activity. Res J Biotechnol 11(9):75–78
Favvas EP, Tsanaktsidis CG, Sapalidis AA et al (2016) Clinoptilolite, a natural zeolite material: structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater 255:385–391
Kussainova MZ, Chernyakova RM, Jussipbekov UZ, Paşa S (2019) Structural investigation of raw clinoptilolite over the Pb2+ adsorption process from phosphoric acid. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.02.012
Miraboutalebi SM, Nikouzad SK, Peydayesh M et al (2017) Methylene blue adsorption via maize silk powder: kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf Environ Prot 106:191–202
Wan M-W, Kan C-C, Rogel BD, Dalida MLP (2010) Adsorption of copper(II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr Polym 80:891–899
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41562011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, T., Zhang, W., Liu, H. et al. Enhanced removal of U(VI) from aqueous solution by chitosan-modified zeolite. J Radioanal Nucl Chem 323, 1003–1012 (2020). https://doi.org/10.1007/s10967-019-06993-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06993-w