Abstract
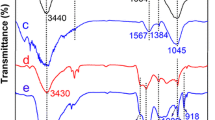
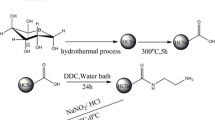
The carbon microsphere which possesses a large number of carboxyl groups on its surface was synthesized by hydrothermal treatment from aqueous glucose solutions at 130 °C and further calcination. The results showed that the maximum adsorption capacity (163 mg/g) of the carbon microspheres towards U(VI) was obtained, and the adsorption kinetic was well fitted by the pseudo-second-order kinetic model. Meanwhile, the adsorption isotherm was better described by the Langmuir model. The thermodynamic parameters indicated the adsorption process was an endothermic and spontaneous process. The possible adsorption mechanism of the carbon microspheres for U(VI) involves ion-exchange and coordination reaction.














Similar content being viewed by others
References
Donia AM, Atia AA, Moussa EM, El-Sherif AM, El-Magied MOA (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95(3):183–189
Clark MW, Harrison JJ, Payne TE (2011) The pH-dependence and reversibility of uranium and thorium binding on a modified bauxite refinery residue using isotopic exchange techniques. J Colloid Interface Sci 356(2):699–705
Liu YH, Wang YQ, Zhang ZB, Cao XH, Nie WB, Li Q, Hua R (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Fasfous II, Dawoud JN (2012) Uranium(VI) sorption by multiwalled carbon nanotubes from aqueous solution. Appl Surf Sci 259:433–440
Wang YQ, Zhang ZB, Liu YH, Cao XH, Liu YT, Li Q (2012) Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem Eng J 198:246–253
Mehta VS, Maillot F, Wang Z, Catalano JG, Giammar DE (2014) Effect of co-solutes on the products and solubility of uranium(VI) precipitated with phosphate. Chem Geol 364:66–75
Bertrand M, Plasari E, Lebaigue O, Baron P, Lamarque N, Ducros F (2012) Hybrid LES–multizonal modelling of the uranium oxalate precipitation. Chem Eng Sci 77:95–104
Zhou C, Ontiveros-Valencia A, de Saint Cyr LC, Zevin AS, Carey SE, Krajmalnik-Brown R, Rittmann BE (2014) Uranium removal and microbial community in a H2-based membrane biofilm reactor. Water Res 64:255–264
Hoyer M, Zabelt D, Steudtner R, Brendler V, Haseneder R, Repke JU (2014) Influence of speciation during membrane treatment of uranium contaminated water. Sep Purif Technol 132:413–421
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134(39):16441–16446
Bartosiewicz I, Chwastowska J, Polkowska-Motrenko H (2015) Fractionation studies of trace elements in Polish uranium-bearing geological materials: potential environmental impact. Int J Environ Anal Chem 95(2):121–134
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO2 2+ from aqueous solution. J Phys Chem B 113(4):860–864
Zhang W, Ye G, Chen J (2016) New insights into the uranium adsorption behavior of mesoporous SBA-15 silicas decorated with alkylphosphine oxide ligands. RSC Adv 6(2):1210–1217
Valizadeh S, Younesi H, Bahramifar N (2016) Highly mesoporous K2CO3 and KOH/activated carbon for SDBS removal from water samples: batch and fixed-bed column adsorption process. Environ Nanotechnol Monit Manag 6:1–13
Yang J, Yu M, Chen W (2015) Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: kinetics, equilibrium and thermodynamics. J Ind Eng Chem 21:414–422
Chen L, Ji T, Mu L, Shi Y, Brisbin L, Guo Z, Zhu J (2016) Facile synthesis of mesoporous carbon nanocomposites from natural biomass for efficient dye adsorption and selective heavy metal removal. RSC Adv 6(3):2259–2269
Harris PJ (2007) Solid state growth mechanisms for carbon nanotubes. Carbon 45(2):229–239
Nie BW, Zhang ZB, Cao XH, Liu YH, Liang P (2013) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295(1):663–670
Xu C, Wang J, Yang T, Chen X, Liu X, Ding X (2015) Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr Polym 121:79–85
Ilaiyaraja P, Deb AKS, Sivasubramanian K, Ponraju D, Venkatraman B (2013) Adsorption of uranium from aqueous solution by PAMAM dendron functionalized styrene divinylbenzene. J Hazard Mater 250:155–166
Anirudhan TS, Deepa JR (2015) Synthesis and characterization of multi-carboxyl-functionalized nanocellulose/nanobentonite composite for the adsorption of uranium(VI) from aqueous solutions: kinetic and equilibrium profiles. Chem Eng J 273:390–400
Anirudhan TS, Nima J, Divya PL (2015) Adsorption and separation behavior of uranium(VI) by 4-vinylpyridine-grafted-vinyltriethoxysilane-cellulose ion imprinted polymer. J Environ Chem Eng 3(2):1267–1276
Bai C, Zhang M, Li B, Tian Y, Zhang S, Zhao X, Li S (2015) Three novel triazine-based materials with different O/S/N set of donor atoms: one-step preparation and comparison of their capability in selective separation of uranium. J Hazard Mater 300:368–377
Zhuang ZH, Yang ZG (2009) Preparation and characterization of colloidal carbon sphere/rigid polyurethane foam composites. J Appl Polym Sci 114(6):3863–3869
Ryu J, Suh YW, Suh DJ, Ahn DJ (2010) Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon 48(7):1990–1998
Aydıncak K, Yumak T, Sınag A, Esen B (2012) Synthesis and characterization of carbonaceous materials from saccharides (glucose and lactose) and two waste biomasses by hydrothermal carbonization. Ind Eng Chem Res 51(26):9145–9152
Demir-Cakan R, Baccile N, Antonietti M, Titirici MM (2009) Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem Mater 21(3):484–490
Makowski P, Cakan RD, Antonietti M, Goettmann F, Titirici MM (2008) Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chem Commun 8:999–1001
Guo SR, Gong JY, Jiang P, Wu M, Lu Y, Yu SH (2008) Biocompatible, luminescent silver@ phenol formaldehyde resin core/shell nanospheres: large-scale synthesis and application for in vivo bioimaging. Adv Funct Mater 18(6):872–879
Zhang ZB, Liu YH, Cao XH, Liang P (2013) Sorption study of uranium on carbon spheres hydrothermal synthesized with glucose from aqueous solution. J Radioanal Nucl Chem 295(3):1775–1782
Liu S, Sun J, Huang Z (2010) Carbon spheres/activated carbon composite materials with high Cr(VI) adsorption capacity prepared by a hydrothermal method. J Hazard Mater 173(1):377–383
Song Q, Ma L, Liu J, Bai C, Geng J, Wang H, Li S (2012) Preparation and adsorption performance of 5-azacytosine-functionalized hydrothermal carbon for selective solid-phase extraction of uranium. J Colloid Interface Sci 386(1):291–299
Sakaki T, Shibata M, Miki T, Hirosue H, Hayashi N (1996) Reaction model of cellulose decomposition in near-critical water and fermentation of products. Bioresour Technol 58(2):197–202
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem-Eur J 15(16):4195–4203
Ibarra J, Munoz E, Moliner R (1996) FTIR study of the evolution of coal structure during the coalification process. Org Geochem 24(6):725–735
Lua AC, Yang T (2004) Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J Colloid Interface Sci 274(2):594–601
Chen Z, Ma L, Li S, Geng J, Song Q, Liu J, Li S (2011) Simple approach to carboxyl-rich materials through low-temperature heat treatment of hydrothermal carbon in air. Appl Surf Sci 257(20):8686–8691
Aroua MK, Leong SPP, Teo LY, Yin CY, Daud WMAW (2008) Real-time determination of kinetics of adsorption of lead(II) onto palm shell-based activated carbon using ion selective electrode. Bioresour Technol 99(13):5786–5792
Zhao Y, Liu C, Feng M, Chen Z, Li S, Tian G, Li S (2010) Solid phase extraction of uranium(VI) onto benzoylthiourea-anchored activated carbon. J Hazard Mater 176(1):119–124
Sekar M, Sakthi V, Rengaraj S (2004) Kinetics and equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J Colloid Interface Sci 279(2):307–313
Dolatyari L, Yaftian MR, Rostamnia S (2016) Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J Environ Manag 169:8–17
Kilislioglu A, Bilgin B (2003) Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl Radiat Isot 58(2):155–160
Bhatnagar A, Jain AK (2005) A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci 281(1):49–55
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296(2):434–441
Xu Y, Zondlo JW, Finklea HO, Brennsteiner A (2000) Electrosorption of uranium on carbon fibers as a means of environmental remediation. Fuel Process Technol 68(3):189–208
Li YH, Ding J, Luan Z, Di Z, Zhu Y, Xu C, Wei B (2003) Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 41(14):2787–2792
Moreno-Castilla C, Álvarez-Merino MA, Pastrana-Martínez LM, López-Ramón MV (2010) Adsorption mechanisms of metal cations from water on an oxidized carbon surface. J Colloid Interface Sci 345(2):461–466
Yuan LY, Liu YL, Shi WQ, Li ZJ, Lan JH, Feng YX, Chai ZF (2012) A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15. J Mater Chem 22(33):17019–17026
Guvendiren M, Messersmith PB, Shull KR (2007) Self-assembly and adhesion of DOPA-modified methacrylic triblock hydrogels. Biomacromolecules 9(1):122–128
Acknowledgements
This work was supported by Engineering Research Center for Biomass Materials, Ministry of Education, China (12zxbk08) and Postgraduate Innovation Fund Project by Southwest University of Science and Technology (14ycx015). Thanks for the technology support of Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, H., Lin, X., Qin, Y. et al. Hydrothermal synthesis of carbon microsphere from glucose at low temperature and its adsorption property of uranium(VI). J Radioanal Nucl Chem 311, 695–706 (2017). https://doi.org/10.1007/s10967-016-5106-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5106-9