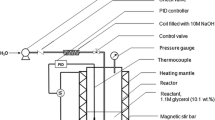
Abstract
Ferric chloride (FeCl3) was used to catalyze the methanolysis of poly(lactic acid) (PLA) to recover methyl lactate. The effects of experimental parameters such as reaction temperature, reaction time, amount of FeCl3 and dosage of methanol on results were studied in detail. The results showed that under the optimized reaction conditions of n(methanol):n(PLA) = 5:1, n(FeCl3):n(PLA) = 0.01:1, 130 °C and 4.0 h, the conversion of PLA reached 96.0 % and the yield of methyl lactate was up to 87.2 %. FeCl3 could be repeatedly used at least 6 times without significant decrease in the conversion of PLA and yield of methyl lactate under the given conditions. The kinetics of the reaction was also investigated. The results indicated that the methanolysis of PLA catalyzed by FeCl3 was a first-order kinetic reaction with activation energy of 32.41 kJ/mol.










Similar content being viewed by others
References
Goswami J, Bhatnagar N, Mohanty S, Ghosh AK (2013) Processing and characterization of poly(lactic acid) based bioactive composites for biomedical scaffold application. Expr Polym Lett 7(7):767–777
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101(22):8493–8501
Aoki S, Kinoshita M, Miyazaki H, Saito A, Fujie T, Iwaya K, Takeoka S, Saitoh D (2013) Application of poly-L-lactic acid nanosheet as a material for wound dressing. Plast Reconstr Surg 131(2):236–240
Frackowiak S, Ludwiczak J, Kozlowski M (2014) Multifunctionality of foamed poly(lactid acid) and its composites with nanofillers. Polym Compos. doi:10.1002/pc.23073
Lai SM, Lan YC (2013) Shape memory properties of melt-blended polylactic acid (PLA)/thermoplastic polyurethane (TPU) bio-based blends. J Polym Res 20(5):1–8
Peng CR, Chen H, Wang JX, Chen ZP, Ni MJ, Chen YH, Zhang J, Yuan T (2013) Controlled degradation of polylactic acid grafting N-vinyl pyrrolidone induced by gamma ray radiation. J Appl Polym Sci 130(1):704–709
Santana VT, Goncalves SPC, Agnelli JAM, Martins-Franchetti SM (2012) Biodegradation of a polylactic acid/polyvinyl chloride blend in soil. J Appl Polym Sci 125(1):536–540
Tabi T, Tamas J, Kovacs G (2013) A new perspective in reinforcing poly(lactic acid) to produce injection moulded engineering composites from renewable and natural resources. Expr Polym Lett 7(2):107–119
Piemonte V, Sabatini S, Gironi F (2013) Chemical recycling of PLA: a great opportunity towards the sustainable development? J Polym Environ 21(3):640–647
Philippe C, Jean-Christophe B, Willocq J (2012) Chemical recycling of PLA by hydrolysis. US 20120142958
Philippe C, Jean-Christophe B, Willocq J (2012) Chemical recycling of PLA by alcoholysis. US 20120029228
Shih YF, Huang CC (2011) Polylactic acid (PLA)/banana fiber (BF) biodegradable green composites. J Polym Res 18(6):2335–2340
Hidayah A, Nishida H, Hassan MA, Shirai Y (2010) Chemical recycling of polyhydroxyalkan-oates as a method towards sustainable development. Biotechnol J 5(5):484–492
Undri A, Rosi L, Frediani M, Frediani P (2014) Conversion of poly(lactic acid) to lactide via microwave assisted pyrolysis. J Anal Appl Pyrol 110:55–65
Badia JD, Santonja-Blasco L, Martinez-Felipe A, Ribes-Greus A (2012) A methodology to assess the energetic valorization of bio-based polymers from the packaging industry: pyrolysis of reprocessed polylactide. Bioresour Technol 111:145–147
Tsukegi T, Yanagida H, Yamashiro K, Shirai Y, Nishida H (2013) Kinetic analysis of thermal degradation of poly-L-lactic acid/ABS blends for selective chemical recycling. Kobun Ronbun 70(10):581–588
Tsukegi T, Motoyama T, Shirai Y, Nishida H, Endo T (2007) Racemization behavior of L, L-lactide during heating. Polym Degrad Stab 92(4):552–559
Piemonte V, Gironi F (2013) Lactic acid production by hydrolysis of poly(lactic acid) in aqueous solutions: an experimental and kinetic study. J Polym Environ 21(1):275–279
Qu M, Tu HL, Amarante M, Song YQ, Zhu SS (2014) Zinc oxide nanoparticles catalyze rapid hydrolysis of poly(lactic acid) at low temperatures. J Appl Polym Sci. doi:10.1002/app.40287
Codari F, Lazzari S, Soos M, Storti G, Morbidelli M, Moscatelli D (2012) Kinetics of the hydrolytic degradation of poly(lactic acid). Polym Degrad Stab 97(11):2460–2466
Plichta A, Lisowska P, Kundys A, Zychewicz A, Debowski M, Florjanczyk Z (2014) Chemical recycling of poly(lactic acid) via controlled degradation with protic (macro)molecules. Polym Degrad Stab 108:288–296
Guo C, Xiang MM, Dong YS (2015) Surface modification of poly(lactic acid) with an improved alkali-acid hydrolysis method. Mater Lett 140(1):144–147
Grala A, Ejfler J, Jerzykiewicz LB, Sobota P (2011) Chemoselective alcoholysis of lactide mediated by a magnesium catalyst: an efficient route to alkyl lactyllactate. Dalton Trans 40:4042–4044
Hirao K, Nakatsuchi Y, Ohara H (2010) Alcoholysis of poly(L-lactic acid) under microwave irradiation. Polym Degrad Stab 95(6):925–928
Philippe C, Jean-Christophe B, Willocq J (2013) Chemical recycling of PLA by alcoholysis. US 8481675B2
Sánchez AC, Collinson SR (2011) The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur Polym J 47(10):1970–1976
Song XY, Liu FS, Li L, Yang XQ, Yu ST, Ge XP (2013) Hydrolysis of polycarbonate catalyzed by ionic liquid [Bmim][Ac]. Journal of Hazardous Materials, J Hazard Mater 244–245:204–208
Liu SW, Wang ZP, Li L, Yu ST, Xie CX, Liu FS (2013) Butanol alcoholysis reaction of polyethylene terephthalate using acidic ionic liquid as catalyst. J Appl Polym Sci 130(3):1840–1844
Song XY, Wang H, Zheng XD, Liu FS, Yu ST (2014) Methanolysis of poly (lactic acid) using acidic functionalized ionic liquids as catalysts. J Appl Polym Sci. doi:10.1002/app.40817
Song XY, Wang H, Yang XQ, Liu FS, Yu ST, Liu SW (2014) Hydrolysis of poly (lactic acid) into calcium lactate using ionic liquid [Bmim][OAc] for chemical recycling. Polym Degrad Stab 110:65–70
Acknowledgments
This research was supported financially by the National Natural Science Foundation of China (No. 21176130), the Doctoral Fund of Ministry of Education (No. 2013379110004) and a project of Shandong Province higher Educational Science and Technology Program (No. J14LC12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, H., Song, X., Liu, F. et al. Ferric chloride as an efficient and reusable catalyst for methanolysis of poly(lactic acid) waste. J Polym Res 22, 135 (2015). https://doi.org/10.1007/s10965-015-0783-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0783-6