Abstract
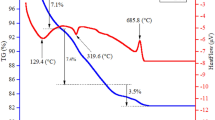
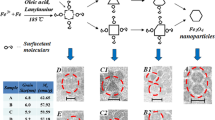
Due to the strong effect of nanoparticles’ size on the magnetic and structural properties of Fe3O4 (magnetite) nanoparticles, the size selection proportional to desired magnetization especially superparamagnetic characteristic of these particles is very important. In this work, at first, the Fe3O4 nanoparticles successfully synthesized by a novel precipitating agent, ethylenediamine (EN), with an ultrasonic treatment (40 kHz, 150 W) by the co-precipitation method. Then, in order to accurately investigate the synthesis conditions on the physical properties of Fe3O4, the influence of reaction temperature, reaction time, and precipitating agent are studied. The structural and magnetic properties of the as-prepared nanoparticles are characterized by X-ray diffraction (XRD), Rietveld refinement, Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), and vibrating sample magnetometer (VSM) analysis. It is found that the EN produces magnetite nanoparticles with a larger size and desired saturation magnetization (Ms). The absence of impurity phases in all of the synthesized nanoparticles and formation of the spinel structures at low temperature (30 °C) can be due to the influence of the ultrasound waves.











Similar content being viewed by others
References
Ding, C., et al.: Controllable synthesis of Fe3O4 polyhedron possessing excellent high-rate electrochemical performance for lithium-ion batteries. Mater. Res. Bull. 97, 142–149 (2018)
Singh, P., Upadhyay, C.: Role of silver nanoshells on structural and magnetic behavior of Fe3O4 nanoparticles. J. Magn. Magn. Mater. 458, 39–47 (2018)
Khmelinskii, I., Makarov, V.: EPR hyperthermia of S. cerevisiae using superparamagnetic Fe3O4 nanoparticles. J. Therm. Biol. 77, 55–61 (2018)
Aghazadeh, M., Karimzadeh, I., Ganjali, M.R., Morad, M.M.: A novel preparation method for surface coated superparamagnetic Fe3O4 nanoparticles with vitamin C and sucrose. Mater. Lett. 196, 392–395 (2017)
Abazari, R., et al.: The effect of different parameters under ultrasound irradiation for synthesis of new nanostructured Fe3O4@ bio-MOF as an efficient anti-leishmanial in vitro and in vivo conditions. Ultrason. Sonochem. 43, 248–261 (2018)
Sriram, B., Govindasamy, M., Wang, S.-F., Ramalingam, R.J., Al-lohedan, H., Maiyalagan, T.: Novel sonochemical synthesis of Fe3O4 nanospheres decorated on highly active reduced graphene oxide nanosheets for high sensitive detection of uric acid in biological samples. Ultrasonics Sonochem. 104618 (2019)
Hashemi, H., Namazi, H.: Sonochemically synthesized blue fluorescent functionalized graphene oxide as a drug delivery system. Ultrason. Sonochem. 42, 124–133 (2018)
Sanaeifar, N., Rabiee, M., Abdolrahim, M., Tahriri, M., Vashaee, D., Tayebi, L.: A novel electrochemical biosensor based on Fe3O4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose. Anal. Biochem. 519, 19–26 (2017)
Boustani, K., Shayesteh, S.F., Salouti, M., Jafari, A., Shal, A.A.: Synthesis, characterisation and potential biomedical applications of magnetic core–shell structures: carbon-, dextran-, SiO2-and ZnO-coated Fe3O4 nanoparticles. IET Nanobiotechnol. 12(1), 78–86 (2017)
Ganesan, V., Lahiri, B., Louis, C., Philip, J., Damodaran, S.P.: Size-controlled synthesis of superparamagnetic magnetite nanoclusters for heat generation in an alternating magnetic field. J. Mol. Liq. 281, 315–323 (2019)
Mascolo, M., Pei, Y., Ring, T.: Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials. 6(12), 5549–5567 (2013)
Snoussi, Y., Bastide, S., Abderrabba, M., Chehimi, M.M.: Sonochemical synthesis of Fe3O4@ NH2-mesoporous silica@ Polypyrrole/Pd: a core/double shell nanocomposite for catalytic applications. Ultrason. Sonochem. 41, 551–561 (2018)
Zhang, X., Zhou, R., Rao, W.: S. P. C. Shanghai 201800 PR China, and S. P. Shanghai 201800 PR C, Influence of precipitator agents NaOH and NH4OH on the preparation of Fe3O4 nano-particles synthesized by electron beam irradiation. J. Radioanal. Nucl. Chem. 270(2), 285–289 (2006)
Peternele, W.S., et al.: Experimental investigation of the coprecipitation method: an approach to obtain magnetite and maghemite nanoparticles with improved properties. J. Nanomater. 2014, 94 (2014)
Wu, X., Xu, G., Zhu, J.-J.: Sonochemical synthesis of Fe3O4/carbon nanotubes using low frequency ultrasonic devices and their performance for heterogeneous sono-persulfate process on inactivation of Microcystis aeruginosa. Ultrasonics Sonochemistry. 104634 (2019)
Balachandramohan, J., Anandan, S., Sivasankar, T.: A simple approach for the sonochemical synthesis of Fe3O4-guargum nanocomposite and its catalytic reduction of p-nitroaniline. Ultrason. Sonochem. 40, 1–10 (2018)
W. k., L. D., L. W., and Z. K: One-pot sonochemical synthesis of magnetite@ reduced graphene oxide nanocomposite for high performance Li ion storage. Ultrasonics Sonochem. 45, 167–172 (2018)
Almessiere, M., et al.: Structural, optical and magnetic properties of Tm3+ substituted cobalt spinel ferrites synthesized via sonochemical approach. Ultrason. Sonochem. 54, 1–10 (2019)
Almessiere, M., et al.: Structural, magnetic, optical properties and cation distribution of nanosized Co0. 7Zn0. 3TmxFe2-xO4 (0.0≤ x≤ 0.04) spinel ferrites synthesized by ultrasonic irradiation. Ultrasonics Sonochem. 104638 (2019)
Almessiere, M., et al.: Sonochemical synthesis of Eu3+ substituted CoFe2O4 nanoparticles and their structural, optical and magnetic properties. Ultrasonics Sonochem. 104621 (2019)
Wei, R., Lv, X., Yang, M., Xu, J., You, Z.: Improving the property of calcium ferrite using a sonochemical method. Ultrason. Sonochem. 43, 110–113 (2018)
Mahdiani, M., Soofivand, F., Salavati-Niasari, M.: Investigation of experimental and instrumental parameters on properties of PbFe12O19 nanostructures prepared by sonochemical method. Ultrason. Sonochem. 40, 271–281 (2018)
Abbas, M., et al.: Size-controlled high magnetization CoFe2O4 nanospheres and nanocubes using rapid one-pot sonochemical technique. Ceram. Int. 40(2), 3269–3276 (2014)
Mirzaee, S., Shayesteh, S.F.: Ultrasound induced strain in ultrasmall CoFe2O4@ polyvinyl alcohol nanocomposites. Ultrason. Sonochem. 40, 583–586 (2018)
Zinatloo-Ajabshir, S., Salavati-Niasari, M.: Novel poly (ethyleneglycol)-assisted synthesis of praseodymium oxide nanostructures via a facile precipitation route. Ceram. Int. 41(1), 567–575 (2015)
Jafari, A., Boustani, K., Shayesteh, S.F.: Effect of carbon shell on the structural and magnetic properties of Fe 3 O 4 superparamagnetic nanoparticles. J. Supercond. Nov. Magn. 27(1), 187–194 (2014)
Jafari, A., Shayesteh, S.F., Salouti, M., Boustani, K.: Dependence of structural phase transition and lattice strain of Fe3O4 nanoparticles on calcination temperature. Indian J. Phys. 89(6), 551–560 (2015)
Nirouei, M., Jafari, A., Boustani, K.: Magnetic and structural study of FeNi 3 nanoparticles: effect of calcination temperature. J. Supercond. Nov. Magn. 27(12), 2803–2811 (2014)
Ahmad, S., Riaz, U., Kaushik, A., Alam, J.: Soft template synthesis of super paramagnetic Fe 3 O 4 nanoparticles a novel technique. J. Inorg. Organomet. Polym. Mater. 19(3), 355–360 (2009)
Kalyani, S., Sangeetha, J., Philip, J.: Effect of precipitating agent and solvent polarity on the size and magnetic properties of magnetite nanoparticles prepared by microwave assisted synthesis. J. Nanosci. Nanotechnol. 16(9), 9591–9602 (2016)
Zhang, D., Zheng, J., Tong, Z.: Fabrication and characterisation of Fe3O4 nanowires via an ethylenediamine-assisted route. J. Exp. Nanosci. 5(2), 162–168 (2010)
Ayyappan, S., et al.: Effect of initial particle size on phase transformation temperature of surfactant capped Fe3O4 nanoparticles. J. Appl. Phys. 109(8), 084303 (2011)
Millan, A., et al.: Surface effects in maghemite nanoparticles. J. Magn. Magn. Mater. 312(1), L5–L9 (2007)
Kirillov, V., et al.: Dimethylsulfoxide as a media for one-stage synthesis of the Fe3O4-based ferrofluids with a controllable size distribution. Mater. Chem. Phys. 225, 292–297 (2019)
Feng, J., Mao, J., Wen, X., Tu, M.: Ultrasonic-assisted in situ synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J. Alloys Compd. 509(37), 9093–9097 (2011)
Wu, J.-H., Ko, S.P., Liu, H.-L., Kim, S., Ju, J.-S., Kim, Y.K.: Sub 5 nm magnetite nanoparticles: synthesis, microstructure, and magnetic properties. Mater. Lett. 61(14–15), 3124–3129 (2007)
Lin, C.-C., Ho, J.-M., Wu, M.-S.: Continuous preparation of Fe3O4 nanoparticles using a rotating packed bed: dependence of size and magnetic property on temperature. Powder Technol. 274, 441–445 (2015)
Oh, J.-M., Hwang, S.-H., Choy, J.-H.: The effect of synthetic conditions on tailoring the size of hydrotalcite particles. Solid State Ionics. 151(1–4), 285–291 (2002)
Lee, J., Isobe, T., Senna, M.: Preparation of ultrafine Fe3O4Particles by precipitation in the presence of PVA at high pH. J. Colloid Interface Sci. 177(2), 490–494 (1996)
Hesani, M., Yazdani, A., Ravan, B.A., Ghazanfari, M.: The effect of particle size on the characteristics of FeCo nanoparticles. Solid State Commun. 150(13–14), 594–597 (2010)
Šutka, A., et al.: Study of the structural phase transformation of iron oxide nanoparticles from an Fe2+ ion source by precipitation under various synthesis parameters and temperatures. Mater. Chem. Phys. 149, 473–479 (2015)
Acknowledgments
The authors gratefully acknowledge the University of Guilan for financial and facility support.
Funding
This work is financially supported by the University of Guilan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boustani, K., Shokri, A., Shayesteh, S.F. et al. Ultrasound-Assisted Synthesis and Tuning the Magnetic and Structural Features of Superparamagnetic Fe3O4 Nanoparticles by Using Ethylenediamine as a Precipitating Agent. J Supercond Nov Magn 33, 1879–1887 (2020). https://doi.org/10.1007/s10948-020-05436-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05436-y