Abstract
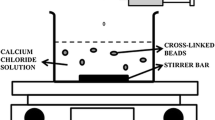
This study was developed to improve the durability and bioavailability of polymer matrix containing the antibacterial agent’s bacitracin (BAC), berberine (BER) and sodium nitroprusside (SNP), an ionotropic gelation method was successfully applied to prepare alginate/chitosan core–shell beads. The structure and properties of different core–shell beads were characterized by scanning electron microscopy (SEM), zeta potential, Fourier transform infrared spectroscopy (FTIR), thermal analysis (DSC), swelling tests, bioadhesive assay, release kinetic profile and antibacterial/antibacteriostatic activity in bacterial model. The morphology of beads was investigated by SEM, display relatively spherical shape and revealed rough surface was also observed the appearance of cracks probably caused by partial collapsing of the polymer network during drying. The diameter means particle size observed was around 1.04 ± 0.13 mm, the particles showed a neutral value, around + 0.305 mV. Using UV–Vis technique was observed a high entrapment efficiency of compounds BAC, BER and SNP (> 95%) in the alginate/chitosan core–shell beads. As demonstrated, there is increase in the swelling degree in pH 6.2. The drug release profile showed a pH-dependent release kinetics. At pH 5.0 the most suitable kinetic model is Higuchi, at pH 6.2 a zero-order model is observed, while at pH 7.4 the Korsmeyer–Peppas model present a good fit. The presence of compounds on beads was confirmed using FTIR analyses, and the results indicated that there is no interaction between drugs and vehicle used in the formulation. We can consider after DSC analysis that beads containing BAC, BER and SNP are thermally more stable than separate formulations having the characteristics required for application at room temperature. The release system produced has physical characteristics that allow the storage of the drugs for long periods of time maintaining their chemical and pharmacological properties unchanged. The percentage of adhesion displayed value of 66%, that indicates the improvement in adherence time on the absorbing surfaces to improves drug bioavailability and effectiveness of compounds. In this study is displayed the additive effect between BAC, BER and SNP shown the potential application of compound combinations as an efficient, novel therapeutic tool for antibiotic-resistant bacterial infections. These results indicate that the proposed strategy improves drug bioavailability and effectiveness of compounds in the treatment of fish.







Similar content being viewed by others
References
Kotob MH, Menanteau-Ledouble S, Kumar G, Abdelzaher M, El-Matbouli M (2016) Vet Res 47(1):98
Cheung RCF, Wong JH, Pan WL, Chan YS, Yin CM, Dan XL, Wang HX, Fang EF, Lam SK, Ngai PHK, Xia LX, Liu F, Ye XY, Zhang GQ, Liu QH, Sha O, Lin P, Ki C, Bekhit AA, Bekhit AED, Wan DCC, Ye XJ, Xia J, Ng TB (2014) Appl Microbiol Biotechnol 98(8):3475–94
Assefa A, Abunna F (2018) Vet Med Int 2018(5432):497
Costa P, Lobo JMS (2001) Eur J Pharm Sci 13(2):123–33
Mutoloki S, Munang’andu HM, Evensen O (2015) Front Immunol 6:519
de Moraes FR, Santos DMS (2010) Qualidade da água e histopatologia de órgãos de peixes provenientes de criatórios do município de Itapecuru Mirim, Maranhão
Carraschi SP, Cruz C, Neto JGM, Castro MP, Bortoluzzi NL (2011) GÃrio ACF. Arq Bras de Med Vet e Zootec 63:579–583
Ciesiolka J, Jezowska-Bojczuk M, Wrzesinski J, Stokowa-Soltys K, Nagaj J, Kasprowicz A, Blaszczyk L, Szczepanik W (2014) Biochim Biophys Acta 1840(6):1782–9
Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dolz H, Millanao A, Buschmann AH, Support NUGR (2013) Environ Microbiol 15(7):1917–42
Johnson BA, Anker H, Meleney FL (1945) Science 102(2650):376–377
Ming LJ, Epperson JD (2002) J Inorg Biochem 91(1):46–58
Hong W, Gao X, Qiu P, Yang J, Qiao M, Shi H, Zhang D, Tian C, Niu S, Liu M (2017) Int J Nanomed 12:4691
Venkateswerlu G (1981) J Biosci 3(1):1–5
Chen ZJ, Chen JX, Wu LK, Li BY, Tian YF, Min X, Huang ZP, Yu RA (2019) Biomed Environ Sci 32(1):1–10
Bahar M, Deng Y, Zhu X, He S, Pandharkar T, Drew ME, Navarro-Vazquez A, Anklin C, Gil RR, Doskotch RW, Werbovetz KA, Kinghorn AD (2011) Bioorg Med Chem Lett 21(9):2606–10
Bellmann R, Smuszkiewicz P (2017) Infection 45(6):737–779
Bhattarai R, Dhandapani N, Shrestha A (2011) Chron Young Sci 2(4):192–196
Imenshahidi M, Hosseinzadeh H (2016) Phytother Res 30(11):1745–1764
Boateng JS, Ayensu I (2014) Drug Dev Ind Pharm 40(5):611–8
Bravo-Osuna I, Andres-Guerrero V, Arranz-Romera A, Esteban-Perez S, Molina-Martinez IT, Herrero-Vanrell R (2018) Adv Drug Deliv Rev 126:127–144
Caetano LA, Almeida AJ, Goncalves LMD (2016) Mar Drugs 14(5):90
George M, Abraham TE (2006) J Control Release 114(1):1–14
Guo MQ, Hu X, Wang C, Ai L (2017) Polysaccharides: structure and solubility. In: Xu Z (ed) Solubility of polysaccharides. IntechOpen. https://doi.org/10.5772/intechopen.71570
Chourasia MK, Jain SK (2004) Drug Deliv 11(2):129–48
Smola M, Vandamme T, Sokolowski A (2008) Int J Nanomed 3(1):1–19
Zhang L, Sang Y, Feng J, Li Z, Zhao A (2016) J Drug Target 24(7):579–89
Pardakhty A, Ranjbar M, Moshafi MH, Abbasloo S (2018) J Clust Sci 29(6):1061–1068
Han S, Dwivedi P, Mangrio FA, Dwivedi M, Khatik R, Cohn DE, Si T, Xu RX (2019) Artif Cells Nanomed Biotechnol 47(1):957–967
Andersen T, Bleher S, Flaten GE, Tho I, Mattsson S, Skalko-Basnet N (2015) Mar Drugs 13(1):222–36
Gomes AJ, Lunardi CN, Lunardi LO, Pitol DL, Machado AEH (2008) Micron 39(1):40–4
de Jesus Gomes A, Lunardi CN, Caetano FH, Lunardi LO, da Hora Machado AE (2006) Microsc Microanal 12(5):399–405
Souza CR, Oliveira HR, Pinheiro WM, Biswaro LS, Azevedo RB, Gomes AJ, Lunardi CN (2015) J Biomater Nanobiotechnol 6(01):53
Nagarwal RC, Ridhurkar DN, Pandit JK (2010) AAPS PharmSciTech 11(1):294–303
Arefin P, Hasan I, Reza MS (2016) Springerplus 5(1):691
Waterborg JH, Matthews HR (1984) Methods Mol Biol 1:1–3
Takka S, GÃŒrel A (2010) AAPS PharmSciTech 11(1):460–466
Tahtat D, Mahlous M, Benamer S, Khodja AN, Oussedik-Oumehdi H, Laraba-Djebari F (2013) Int J Biol Macromol 58:160–8
Chavda H, Modhia I, Mehta A, Patel R, Patel C (2013) Biomed Res Int 2013(563):651
Campos J, Varas-Godoy M, Haidar ZS (2017) Nanomedicine (London) 12(5):473–490
Ramadas M, Paul W, Dileep KJ, Anitha Y, Sharma CP (2000) J Microencapsul 17(4):405–11
Yang H, Hua S, Wang W, Wang A (2011) Iran Polym J 20(6):479–490
Francis-Floyd R (2011) Mycobacterial infections of fish. Southern Regional Aquaculture Center, Stoneville
Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F (2006) Nanomedicine 2(1):8–21
Gao X, Wang W, Wei S, Li WC (2009) Zhongguo Zhong Yao Za Zhi 34(21):2695–700
Snyder JD, Walker WA (1987) Int Arch Allergy Appl Immunol 82(3–4):351–6
Tomkiewicz D, Casadei G, Larkins-Ford J, Moy TI, Garner J, Bremner JB, Ausubel FM, Lewis K, Kelso MJ (2010) Antimicrob Agents Chemother 54(8):3219–3224
Yong Y, Kai H, Bao-Shun Z, Xue-Gang LI (2014) Pharmacogn Mag 10(38):97
Boberek JM, Stach J, Good L (2010) PloS ONE 5(10):e13,745
Wang Y, Kheir MM, Chai Y, Hu J, Xing D, Lei F, Du L (2011) PLoS ONE 6(8):e23,495
Sadrearhami Z, Nguyen TK, Namivandi-Zangeneh R, Jung K, Wong EH, Boyer C (2018) J Mater Chem B 6(19):2945–2959
Smith JN, Dasgupta TP (2001) J Inorg Biochem 87(3):165–173
Acknowledgements
The authors acknowledge the financial support from the University of Brasilia, the Coordination for the Improvement of Higher Educational Personnel (CAPES code 001), the Federal District Research Foundation (FAPDF), the Scientific and Technological Development Foundation (FINATEC), program PIQ and the National Council for Scientific and Technological Development (CNPq). A.J.G. and C.N.L. conceived and designed the research. O.A.B (PhD student) and C.C.L (undergraduate student) carried out the experiments, worked on the characterization analyses and helped to write the article. A.J.G was responsible for helping in the characterization tests and for guiding the analyses of physico-chemical data. C.N.L. performed SEM assays. A.J.G. interpreted the results, T.A.C. and H.S.M. helped to perform the biological tests. V.P.M was responsible for support in the antimicrobial assays. O.A.B performed fish antimicrobial assays. A.J.G. and C.N.L wrote the manuscript. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, A.J., Barbizan, O.A., Lessa, C.C. et al. Multidrug Core–Shell Bead: A System for Bacterial Infection Treatment in Fish. J Polym Environ 27, 2395–2407 (2019). https://doi.org/10.1007/s10924-019-01524-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01524-w