Abstract
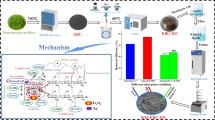
The aim of this study was to synthesize Fe3O4 nanoparticles and gluten/pectin/Fe3O4 composite hydrogel. This nano-hydrogel was then used to reduce sediment contamination in Lake Urmia (Iran). For this purpose, magnetic nanoparticles were chemically synthesized. Two types of composite Hydrogels including gluten/Fe3O4 and gluten/pectin/Fe3O4 composites were prepared by adding Fe3O4 nanoparticles and pectin to wheat gluten. The properties of Hydrogels were studied by scanning electron microscopy (SEM), Fourier-transform infrared and X-ray diffraction methods. SEM results showed that Fe3O4 particles were seed shape (30–60 nm). The results confirmed the physical interactions between magnetic nanoparticles, pectin and gluten. Subsequently, the prepared Hydrogels were used to reduce the pollution of Lake Urmia sediments. Central composite design was used to investigate the effect of interaction time and interaction temperature of hydrogel with sediment and the type of hydrogel. Qualitative characteristics of the sediments, including heavy metal concentrations (arsenic, barium, cadmium, lead, mercury and chromium), biochemical oxygen demand, and chemical oxygen demand COD, and organic compounds were investigated before and after treatment of sediments with hydrogel. The effect of hydrogel on enhancing sediment quality was investigated. The results showed that in the optimum condition removal efficiency of total heavy metals was 62% and removal efficiency of total organic compounds was 42%. The results also showed that gluten/pectin/Fe3O4 hydrogel had better performance in reducing environmental pollutants of sediment than wheat gluten/Fe3O4 hydrogel.





Similar content being viewed by others
References
R. Barzegar, A.A. Moghaddam, S. Soltani, E. Fijani, E. Tziritis, N. Kazemian, Heavy metal (loid) s in the groundwater of Shabestar area (Nw Iran): Source identification and health risk assessment. Water Qual. Exposure Health 11, 251–265 (2017)
M. Monier, D.A. Abdel-Latif, Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg (II), Cd (II) and Zn (II) ions from aqueous solutions. J. Hazard. Mater. 209, 240–249 (2012)
A. Afkhami, M. Saber-Tehrani, H. Bagheri, Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2, 4-dinitrophenylhydrazine. J. Hazard. Mater. 181, 836–844 (2010)
M. Rezaei, S. Pirsa, S. Chavoshizadeh, Photocatalytic/antimicrobial active film based on wheat gluten/ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 39, 1–12 (2019)
H. Wieser, Chemistry of gluten proteins. Food Microbial. 24, 115–119 (2007)
J.M. Krochta, Proteins as raw materials for films and coatings: definitions, current status, and opportunities. Protein-based Films Coat. 1, 1–40 (2002)
M. Ahmad, S. Benjakul, T. Prodpran, T.W. Agustini, Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocol. 28, 189–199 (2012)
J. Shang, Z. Shao, X. Chen, Electrical behavior of a natural polyelectrolyte hydrogel: chitosan/carboxymethylcellulose hydrogel. Biomacromol 9, 1208–1213 (2008)
E. Farshchi, S. Pirsa, L. Roufegarinejad, M. Alizadeh, M. Rezazad, Photocatalytic/biodegradable film based on carboxymethyl cellulose, modified by gelatin and TiO2-Ag nanoparticles. Carbohydr. Polym. 216, 189–196 (2019)
Y. Li, Q. Li, Z. Tan, A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources. 443, 227–262 (2019)
N. Joshi, L.F. Da Silva, H.S. Jadhav, F.M. Shimizu, P.H. Suman, J.C. M’Peko, M.O. Orlandi, J.G. Seo, V.R. Mastelaro, O.N. Oliveira Jr., Yolk-shelled ZnCo2O4 microspheres: surface properties and gas sensing application. Sens. Actuators B 257, 906–915 (2018)
A. Jahanbakhsh, S. Pirsa, M. Bahram, Synthesis and characterization of magnetic nanocomposites based on Hydrogel-Fe3O4 and application to remove of organic dye from waste water. Main Group Chem. 16, 85–94 (2017)
A.K. Hauser, R.J. Wydra, N.A. Stocke, K.W. Anderson, J.Z. Hilt, Magnetic nanoparticles and nanocomposites for remote controlled therapies. J Controll. Release. 219, 76–94 (2015)
A. Da̧browski, Z. Hubicki, P. Podkościelny, E. Robens, Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56, 91–106 (2004)
A. Munitz, I. Edry, E. Brosh, N. Derimow, B.E. MacDonald, E.J. Lavernia, R. Abbaschian, Liquid phase separation in AlCrFeNiMo0. 3 high-entropy alloy. Intermetallics 112, 106517 (2019)
D.V. Pryazhnikov, I.V. Kubrakova, M.S. Kiseleva, L.Y. Martynov, I.Y. Koshcheeva, Preparation and structural characterization of nanosized magnetic solid-phase extractants. Mendeleev Commun. 24, 130–132 (2014)
M. Perez-Mateos, P. Montero, M.C. Gomez-Guillen, Formulation and stability of biodegradable films made from cod gelatinand sunflower oil blends. Food Hydrocol. 23, 53–61 (2009)
S. Pirsa, S. Chavoshizadeh, Design of an optical sensor for ethylene based on nanofiber bacterial cellulose film and its application for determination of banana storage time. Polym. Adv. Technol. 29, 1385–1393 (2018)
N. Alizadeh, A.A. Ataei, S. Pirsa, Nanostructured conducting polypyrrole film prepared by chemical vapor deposition on the interdigital electrodes at room temperature under atmospheric condition and its application as gas sensor. J. Iran Chem. Soc. 12, 1585–1594 (2015)
W. Cai, J. Wan, Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J. Colloid Interface Sci. 305, 366–370 (2007)
D. Maity, P. Chandrasekharan, F. Si-Shen, J.M. Xue, J. Ding, Polyol-based synthesis of hydrophilic magnetite nanoparticles. J. Appl. Phys. 107, 09B310 (2010)
M.D.F. Sato, D.C. Rigoni, M.H.G. Canteri, C.L. Petkowicz, A. Nogueira, G. Wosiacki, Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Sci. Agron. 33, 383–389 (2011)
N. Wellner, D. Bianchini, E.C. Mills, P.S. Belton, Effect of selected Hofmeister anions on the secondary structure and dynamics of wheat prolamins in gluten. Cereal chem. 80, 596–600 (2003)
X. Liu, Q. Hu, Z. Fang, X. Zhang, B. Zhang, Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25, 3–8 (2008)
J. Gong, X. Lin, Facilitated electron transfer of hemoglobin embedded in nano-sized Fe3O4 matrix based on paraffin impregnated graphite electrode and electrochemical catalysis for trichloroacetic acid. Microchem. J. 75, 51–57 (2003)
L. Zhao, H. Mitomo, Adsorption of heavy metal ions from aqueous solution onto chitosan entrapped CM-cellulose hydrogels synthesized by irradiation. J. Appl. Polym. Sci. 110, 1388–1395 (2008)
C. Dong, J. Lu, B. Qiu, B. Shen, M. Xing, J. Zhang, Developing stretchable and graphene-oxide-based hydrogel for the removal of organic pollutants and metal ions. Appl. Catal B. 222, 146–156 (2018)
O. Ozay, S. Ekici, Y. Baran, S. Kubilay, N. Aktas, N. Sahiner, Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environments. Desalination 260, 57–64 (2010)
M.R. Guilherme, A.V. Reis, A.T. Paulino, T.A. Moia, L.H. Mattoso, E.B. Tambourgi, Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J. Appl. Polym. Sci. 117, 3146–3154 (2010)
L.V. Candioti, M.M. De Zan, M.S. Camara, H.C. Goicoechea, Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124, 123–138 (2014)
Acknowledgements
This research was supported by the Urmia Lake Research Institute, Urmia University, Iran (Project No. 98/M/003) whose valuable assistance greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirsa, S., Asadzadeh, F. & Karimi Sani, I. Synthesis of Magnetic Gluten/Pectin/Fe3O4 Nano-hydrogel and Its Use to Reduce Environmental Pollutants from Lake Urmia Sediments. J Inorg Organomet Polym 30, 3188–3198 (2020). https://doi.org/10.1007/s10904-020-01484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01484-y