Abstract
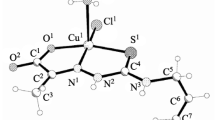
A novel –M–X–M–X– type infinite chain 1D copper(II) complex of Eflornithine, Dichloro-[2-amino-5-ammonio-2-(difluoromethyl)pentanoate]copper(II) hydrate, [Cu(C6H12F2N2O2)Cl2]·H2O 1 has been synthesized and characterized by elemental analysis, spectroscopic techniques (UV/Vis and FT-IR), TGA and X-ray diffraction. Single-crystal X-ray diffraction analysis of the complex 1 showed the structure to be monoclinic with space group Cc, a = 13.1295(15) Å, b = 12.1859(14) Å, c = 8.1927(9) Å, β = 118.359(3) Å, V = 1153.5(2) Å3, Z = 4. The complex exhibits a quadratic planar coordination of the Cu-atom. The Cu(II) centre is coordinated by two chloride atoms, an oxygen atom of the carboxyl- and a nitrogen atom of the amino-group, respectively, forming a quadratic planar geometry. The terminal amino group of the ligand is protonated to form NH3 + while the carboxylic moiety is deprotonated to form Zwitterionic eflornithine ligand, with the coordination of the metal at the nitrogen atom of the second amino group. The compound has –M–X–M–X– infinite 1D chain polymeric structure. Two neigbouring Cu(EFL)Cl chain units are bridged by an Cl− ion, forming a –Cu–Cl–Cu–Cl– linear chain structure along C-axis. The antibacterial activities of the complex on Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa (P. aeruginosa) were investigated and found to be active at higher concentration than the parent ligand.






Similar content being viewed by others
References
G.B. Lundkvist, K. Kinstensson, M. Bentivoglio, Physiology 19, 198 (2004)
E.M. Cornford, W.D. Bocash, L.D. Braun, P.D. Crane, W.H. Oldendorf, A.J. Maclnnis, J. Clin. Investig. 63(6), 1241 (1979)
R. Brun, J. Blum, F. Chappuis, C. Burri, Lancet 375, 148 (2010)
Uganda Sleeping Sickness Reaching Alarming Level. New Vision, 11 May 2008
J. Pepin, F. Milord, A.N. Khonde, T. Nivonsenga, L. Loko, B. Mpia, Trans R. Soc. Trop. Med. Hyg. 89, 92 (1995)
WHO Technical Report Series 881. Geneva (1998)
C. Schmid, M. Richer, C.M. Bilenge, T. Josenando, F. Chappuis, C.R. Manthelot, J. Infect. Dis. 191, 1922 (2005)
D. Legros, S. Evans, F. Maiso, J.C. Enyaru, D. Mbulamberi, Trans R. Soc. Trop. Med. Hyg. 93, 439 (1999)
C. Burri, J. Keiser, Trop. Med. Int. Health. 6, 412 (2001)
A. Stanghellini, T. Josenando, Trop Med. Int. Health. 6, 330 (2001)
B.W. Metcalf, P. Bev, C. Danzin, M.J. Jung, P. Casara, J.P. Vevert, J. Am. Chem. Soc. 100, 2551 (1978)
C.J. Bacchi, H.C. Nathan, S.H. Hutner, P.P. McCann, Science 210, 332 (1980)
C.J. Bacchi, J. Garofalo, D. Mockenhaupt, P.P. McCann, K.A. Diekema, A.E. Pegg, Parasitol. 7, 209 (1983)
R.L. Krauth-Siegel, H. Bauer, R.H. Schirmer, Chem. Int. Ed. 44, 690 (2005)
C. Zhang, S. Lippard, Curr. Opin. Chem. Biol. 7, 481 (2003)
P.A. Ajibade, G.A. Kolawole, M. Brien, P.O. Helliweel, J. Raffery, Inorg. Chim. Acta 395, 3111 (2006)
M. Navarro, J. Med. Chem. 47, 5204 (2004)
J.A. Obaleye, J.B. Nde-aga, J.A. Balogun, Afr. J. Sci. 1, 10 (1997)
J.A. Obaleye, M.R. Caira, A.C. Tella, Struct. Chem. 20, 859 (2009)
A.W. Boh, Curr. Sep. 18(2), 47 (1999)
O. Anderson, J. Aaseth, Environ. Health 110, 887 (2002)
C. Orvig, M.J. Abrams, Chem. Rev. 99(9), 2201 (1999)
P.P. Silva, W. Guerra, J.N. Silveira, A.M.C. Ferreira, T. Bortolotto, F.L. Fischer, H. Terenzi, A. Neves, E.C. Pereira-Maia, Inorg. Chem. 50(14), 6414 (2011)
E. Chalkidov, F. Perdish, I. Turel, P. Kessissoglou, G. Psomas, J. Inorganic Biochem. 113, 55 (2012)
A.C. Tella, J.A. Obaleye, Orbit. Electron. J. Chem. Campo grande, 2(1), 11 (2010)
D. Braga, F. Grepioni, L. Maini, R. Brescello, L. Cotarca, Cryst Eng. Comm. 10, 489 (2008)
P.A. Ajibade, O.G. Idemudia, A.I. Okoh, Bull. Chem. Soc. Ethiop. 27(1), 77 (2013)
D. Superflip, J. Appl. Cryst. 40, 786 (2007)
G.M. Sheldrick, Acta Cryst A64, 112 (2008)
O.V. Dolomanor, L.J. Bourhus, R.J. Gildea, J.A.K. Howard, H. Puschmann, Appl. Cryst. 42, 339 (2009)
NCCLS Performance of Standards for Antimicrobial Susceptibility. 8th International supplement (2006) M100S12
J.A. Obaleye, M.R. Caira, A.C. Tella, J. Chem. Crystallogr. 37, 707 (2007)
R. Hubel, T. Jelinek, W. Beck, Zeitschrift fuer Naturforchung. 55b, 821 (2000)
K.Y. Kim, H.C. Chang, Y.T. Lee, U.I. Cho, D.M. Bow, J. Phys. Chem. 107, 5007 (2003)
X. Renshang, Y. Yang, Z. Veimin, Introduction to Natural Products Chemistry (CRC Press, Taylor and Francis, 2012)
A. Kamariotis, O.V. Boyarkn, S.R. Mercier, R.D. Beck, M.F. Bush, E.R. Williams, T.R. Rizzo, J. Am. Chem. Soc. 128, 905 (2006)
Y. Elizabeth, B.S. Chandravanshi, R.K. Gridasova, Bull. Chem. Soc. Ethiopia 9(1), 1 (1995)
A.C. Ukwueze, Bull. Chem. Soc. Ethiop 7 (1), 61 (1993)
R. Malhotra, S.K. Jyoti, H.R. Singal, K.S. Dhindra, Indian J. Chem. 39A, 421 (2000)
H. Zerrin, K. Cem, C.C. Ersanah, O.Z. Yesilel, O. Buyukgungor, Z. Naturforsh, 61b 1072, (2006)
J.B. Deacon, R. Philip, Coord. Chem. Rev. 23, 227 (1980)
B. Srinnivas, N. Arulsany, P.S. Zacharias, Polyhedron 10, 731 (1999)
C.K. Choudhary, R.K. Choudhary, L.K. Mishia, J. Indian Chem. Soc. 80, 693 (2003)
B.B. Mohapatra, S.K. Saraf, J. Indian Chem. Soc. 80, 696 (2003)
P. Biswas, S. Dutta, M. Ghosh, Polyhedron 27, 2105 (2008)
A.B.P. Lever, Inorganic Electronic Spectroscopy, 2nd edn. (Elsevier, Amsterdam, 1984)
Q-H. Zhao, D-Z. Liao, Z-H. Jiang, S-P. Yan, S.Gao, B-W.Sun, J. Coord. Chem. 55(5), 293 (2002)
G. Tamasi, F. Serinelli, M. Consumi, A. Magnani, M. Casolaro, R. Cini, J. Inorg. Biochem. 102, 1862 (2008)
N. Kulcu, U. Florke, H. Arslan, Turk. J. Chem. 29, 1 (2005)
J.A. Obaleye, M.R. Caira, A.C. Tella, Anal. Sci. 24, x63 (2008)
S. Soralora, M. Breza, Polyhed. 29, 2440 (2010)
H. Behn, Acta Cryst. B38, 2781 (1982)
H. Iguchi, D. Jiang, J. Xie, S. Takasi, M. Yamashita, Polymers 3, 1652 (2011)
I. Hargittai, M. Hargittai, Symmetry Through the Eyes of a Chemist, UCH, 1987
U.N. Ekwenye, N.N. Elegalam, J. Mol. Med. Adv. Sci. 1(4), 410 (2005)
P.F. Omojasola, S. Awe, Biosci. Res. Comm. 16, 25 (2004)
Acknowledgments
We are grateful for the support from the World Bank Science and Technology Education Post-Basic Project (STEP-B), Department of Chemistry University of Ilorin and Dr. Michael Wörle for help with the crystal structure determination during the Zurich School of Crystallography 2013 at the University of Zurich, Institute of Organic Chemistry, Winterthurersthrasse 190 CH-8057, Zurich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obaleye, J.A., Tella, A.C., Osunniran, W.A. et al. Synthesis, Characterization, Crystal Structure and Antimicrobial Evaluation of a Novel –M–X–M–X– Type Infinite Chain 1D Cu(II) Complex with Eflornithine Hydrochloride Hydrate as Ligand. J Inorg Organomet Polym 24, 827–835 (2014). https://doi.org/10.1007/s10904-014-0052-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-014-0052-x