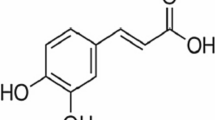
Abstract
The binding of quercetin to lysozyme (LYSO) in aqueous solution was investigated by fluorescence spectroscopy, UV-vis absorption spectroscopy and molecular simulation at pH 7.4. The fluorescence quenching of LYSO by addition of quercetin is due to static quenching, the binding constants, K a , were 3.63 × 104, 3.31 × 104 and 2.85 × 104 L·mol−1 at 288, 298 and 308 K, respectively. The thermodynamic parameters, enthalpy change, ∆H, and entropy change, ∆S, were noted to be −7.56 kJ·mol−1 and 61.07 J·mol−1·K−1. The results indicated that hydrophobic interaction may play a major role in the binding process. The distance r between the donor (LYSO) and acceptor (quercetin) was determined as 3.34 nm by the fluorescence resonance energy transfer. The synchronous fluorescence spectroscopy showed the polarity around the tryptophan residues increased and the hydrophobicity decreased. Furthermore, the study of molecular simulation indicated that quercetin could bind to the active site (a pocket made up of 24 amino-acid residues) of LYSO mainly via hydrophobic interactions and that there were hydrogen interactions between the residues (Gln 57, Ile 98) of LYSO and quercetin. The accessible surface area (ASA) calculation verified the important roles of tryptophan (Trp) residues during the binding process.






Similar content being viewed by others
References
Christine M, Crespy V, Manach C, Besson C, Demigne C, Remesy C (1998) Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol Regul Integr Comp Physiol 275:212–219
Rusznyak ST, Szent-Gyorgyi A (1936) Vitamin P: flavonols as vitamins. Nature 138:27–27
Guardia T, Rotelli AE, Juarez AO, Pelzer LE (2001) Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 56:683–687
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080
Kaldas MI, Walle UK, van der Houde H, McMillan JM, Walle T (2005) Covalent binding of the flavonoid quercetin to human serum albumin. J Agric Food Chem 53:4194–4197
Kanakis CD, Tarantilis PA, Polissiou MG, Diamantoglou S, Tajmir-Riahi HA (2006) Antioxidant flavonoids bind human serum albumin. J Mol Struct 798:69–74
Rawel HM, Frey SK, Meidtner K, Kroll J, Schweigert FJ (2006) Determining the binding affinities of phenolic compounds to proteins by quenching of the intrinsic tryptophan fluorescence. Mol Nutr Food Res 50:705–713
Rawel HM, Meidtner K, Kroll J (2005) Binding of selected phenolic compounds to proteins. J Agr Food Chem 53:4228–4235
Gu Z, Zhu X, Ni S, Su Z, Zhou HM (2004) Conformational changes of lysozyme refolding intermediates and implications for aggregation and renaturation. Int J Biochem Cell Biol 36:795–805
Yang F, Liang L (2003) Unfolding of lysozyme induced by urea and guanidine hydrochloride studied by “phase diagram” method of fluorescence. Acta Chim Sinica 61:803–807
Sanz-Vicente I, Romero JJ, de Marcos S, Ostra M, Ubide C, Galbán J (2009) Simultaneous determination of glucose and choline based on the intrinsic fluorescence of the enzymes. J Fluoresc 19:583–591
Croguennec T, Nau F, Molle D, Le Graet Y, Brule G (2000) Iron and citrate interactions with hen egg white lysozyme. Food Chem 68:29–35
St. Louis, SYBYL software version 6. 9, Tripos Associates Inc., 2002
Delano WL (2004) PyMOL software, version 0.99, USA, San Carlos
Hubbard SJ, Thornton JM (1993) ‘NACCESS’: computer program, Department of Biochemistry and Molecular Biology, University College London
Li DJ, Zhu JF, Jin J, Yao XJ (2007) Studies on the binding of nevadensin to human serum albumin by molecular spectroscopy and simulation. J Mol Struct 846:34–41
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Plenum Press, New York, pp 237–265
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170
Ware WR (1962) Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process. J Phys Chem 66:455–458
Bi SY, Ding L, Tian Y, Song DQ, Zhou X, Liu X et al (2004) Investigation of the interaction between flavonoids and human serum albumin. J Mol Struct 703:37–45
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102
Tian J, Liu J, Hu Z, Chen X (2005) Interaction of wogonin with bovine serum albumin. Bioorg Med Chem 13:4124–4129
Rahman MH, Maruyama T, Okada T, Yamasaki K, Otagiri M (1993) Study of interaction of carprofen and its enantiomers with human serum albumin-I: mechanism of binding studied by dialysis and spectroscopic methods. Biochem Pharmacol 46:1721–1731
Stryer L, Haugland RP (1967) Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci USA 58:719–726
Förster T (1965) In: Sinanoglu O (ed) Modern quantum chemistry, vol. 3. Academic, New York, pp 93–137
Valeur B, Brochon JC (2001) New trends in fluorescence spectroscopy. Springer, Berlin, p 25
Eisinger J, Feuer B, Lamola AA (1969) Intramolecular singlet excitation transfer. Applications to polypeptides. Biochemistry 8:3908–3915
Steinberg IZ (1971) Long-range nonradiative transfer of electronic excitation energy in proteins and polypeptides. Annu Rev Biochem 40:83–114
Burstein EA, Vedenkina NS, Ivkova MN (1973) Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol 18:263–279
Klajnert B, Bryszewska M (2002) Fluorescence studies on PAMAM dendrimers interactions with bovine serum albumin. Bioelectrochemistry 55:33–35
Hu YJ, Liu Y, Pi ZB, Qu SS (2005) Interaction of cromolyn sodium with human serum albumin: a fluorescence quenching study. Bioorg Med Chem 13:6609–6614
Imoto T, Johnson LN, North ACT, Phillips DC, Rupley JA (1972) In: Boyer PD (ed) The enzymes. Academic, New York, pp 666–868
Hayashi K, Imoto T, Funatsu G, Funatsu M (1965) The position of the active tryptophan residue in lysozyme. J Biochem 58:227–235
Rmoso C, Förster LS (1975) Tryptophan fluorescence lifetimes in lysozyme. J Biol Chem 250:3738–3745
He WY, Li Y, Xue CX, Hu ZD, Chen XG, Sheng FL (2005) Effect of Chinese medicine alpinetin on the structure of human serum albumin. Bioorg Med Chem 13:1837–1845
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Grant no. 20673034) and the Research Fund for the Doctoral Program of Higher Education of China (Grant no. 20060476001) for their financial supports. In addition, we thank Lanzhou University for supporting the molecular simulation software (Sybyl 6.9) and SGI FUEL workstations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Wang, L., Tang, W. et al. Binding of Quercetin to Lysozyme as Probed by Spectroscopic Analysis and Molecular Simulation. J Fluoresc 21, 1879–1886 (2011). https://doi.org/10.1007/s10895-011-0884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0884-5