Abstract
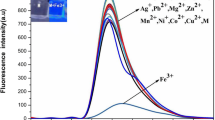
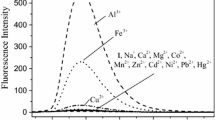
Multicomponent systems 1, 2, and 3 havingfluorophore–spacer–receptor architecture have been prepared with a view to understand the role of the photoinduced intramolecular electron transfer (PIET) interactions in transition metal ion sensing efficiency of these systems. Structurally similar compounds 4-amino-1,8-naphthalimide (4), 4-aminophthalimide (5), and 4-methoxy-1,8-naphthalimide (6) were used as the fluorophore moieties. Dimethylamino group (as in the case of 1a, 2a, and 3a, series A) and an aniline moiety (like in 1b, 2b, and 3b, series B) have been employed as the receptor components. A two-carbon ethylene chain serves as a spacer unit. The absorption and fluorescence spectral features of the systems have been studied in the absence and presence of various transition metal ions. All the multicomponent systems (except 1a) show weak fluorescence intensities compared to that of their constituent fluorophores (4, 5, and 6) in any given solvent. The reason for this low fluorescence quantum yield could be ascribed to the efficient PIET interaction between receptor moiety and the electron deficient fluorophore component of the systems. This has been corroborated with the estimated thermodynamic driving force (Δ G*) for the PIET process in the multicomponent systems, calculated using electrochemical and spectral properties of individual components, is more negative for 2 and 3 than for 1 having electron deficient fluorophores 5, and 6, respectively. Especially, as evidenced by their low fluorescence quantum yield values, the PIET interaction is found to be more significant in the systems of series B (1b, 2b, and 3b) than the respective system of series A (1a, 2a, and 3a, respectively). The sensing capability of the systems is directly related to the efficiency of the PIET interaction in the unbound state. Accordingly, all these systems (except 1a) show significant fluorescence enhancement in the presence of transition metal ions, well known for their high fluorescence quenching behavior. The present paper describes, the feasibility of optimizing the PIET interaction in the multicomponent sensor system in unbound state, and thus transition metal ion signaling capability of the system.
Similar content being viewed by others
REFERENCES
(a) F. L. Carter, R. E. Siatkowski, and H. Wohltjen (Eds.) (1988). Molecular Electronic Devices; Elsevier, Amsterdam. (b) J. M. Lehn (1995). Supramolecular Chemistry; VCH, Weinheim. (c) V. Balzani, A. Juris, A. M. Venturi, S. Campagna, and S. Serroni (1996). Luminescent and redox-active polynuclear transition metal complexes. Chem. Rev. 962, 759–833.
(a) J. P. Desvergene and A. W. Czarnik (Eds.) (1997). Chemosensors of Ion and Molecular Recognition; NATO ASI Series C492, Kluwer Academic, Dordrecht. (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A.J. M. Huxley, C. P. McCoy, J. T. Rademacher, and T. E. Rice (1997). Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 975, 1515–1566. (c) B. Valeur (1994). In J. R. Lakowicz (Ed.). Topics in Fluorescence Spectroscopy, Vol. 4, Plenum Press, New York, p. 21. (d) T. D. James, P. Linnane, and S. Shinkai (1996). Fluorescent saccharide receptors: A sweet solution to the design, assembly and evaluation of boronic acid derived PET sensors. Chem. Commun. 281–288. (e) L. Fabbrizzi, M. Licchelli, P. Pallavicini, and D. Sacchi (1994). An anthracene-based fluorescent sensor for transition metal ions. Angew. Chem. Int. Ed. Engl. 3319, 1975–1977. (f) L. Prodi, F. Bolletta, M. Montalti, and N. Zaccheroni (2000). Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 205, 59–83.
(a) A. W. Czarnik (1994). Chemical communication in water using fluorescent chemosensors.Acc. Chem. Res. 2710, 302–308. (b) L. Fabbrizzi, M. Licchelli, P. Pallavicini, A. Perotti, A. Taglietti, and D. Sacchi (1996). Fluorescent sensors for transition metals based on electron-transfer and energy-transfer mechanisms, Chem. Eur. J. 21, 75–82.
(a) P. Ghosh, P. K. Bharadwaj, S. Mandal, and S. Ghosh (1996). Ni(II), Cu(II), and Zn(II) cryptate-enhanced fluorescence of a trianthrylcryptand: A potential molecular photonic OR operator. J. Am. Chem. Soc. 118, 1553. (b) S. Banthia and A. Samanta (2002). Photophysical and transition metal ion signaling behavior of a three-component system comprising a cryptand moiety as the receptor. J. Phys. Chem. A 106, 5572.
A. W. Varnes, R. B. Dodson, and E. L. Wehry (1972). Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies. J. Am. Chem. Soc. 943, 946–950.
J. A. Kemlo and T. M. Shepherd (1977). Quenching of excited singlet states by metal ions, Chem. Phys. Lett. 471, 158–162.
Ramachandram and A. Samanta (1997). Modulation of metal-fluorophore communication to develop structurally simple fluorescent sensors for transition metal ions, Chem. Commun. (11), 1037–1038.
B. Ramachandram and A. Samanta (1998). How important is the quenching influence of the transition metal ions in the design of fluorescent PET sensors? Chem. Phys. Lett. 290(1–3), 9–16.
B. Ramachandram and A. Samanta (1998). Transition metal ion induced fluorescence enhancement of 4-(N,N-dimethylethylenediamino)-7-nitrobenz-2-oxa-1,3-diazole. J. Phys. Chem. A 102, 10579–10587.
B. Ramachandram, N. B. Sankaran, and A. Samanta (1999). Fluorescence response of structurally simple fluorophore–spacer–receptor systems towards transition metal ions and protons. Res. Chem. Intermed. 259, 843–859.
B. Ramachandram, N. B. Sankaran, R. Karmakar, S. Saha, and A. Samanta (2000). Fluorescence signaling of transition metal ions by multi-component systems comprising 4-chloro-1,8-naphthalimide as fluorophore. Tetrahedron 5636, 7041–7044.
B. Ramachandram, G. Saroja, N. B. Sankaran, and A. Samanta (2000). Unusually high fluorescence enhancement of some 1,8-naphthalimide derivatives induced by transition metal salts. J. Phys. Chem. B 104, 11824.
R. Badugu (2002). Development of efficient fluorosensors for the transition metal ions by tuning of photoinduced intramolecular electron transfer (PIET) communication between the components. Chem. Lett. 52.
D. D. Perrin, W. L. Armarego, and D. R. Perrin (1980). Purification of Laboratory Chemicals, Pergamon Press, New York.
C. Reichardt (1998) Solvents and Solvent Effects in Organic Chemistry; VCH, Weinheim, Chapter 7.
D. V. O’Connor and D. Phillips (1984). Time-Correlated Single Photon Counting; Academic Press, London.
K. Rechthaler and G. Köhler (1994). Excited state properties and deactivation pathways of 7-aminocoumarins. Chem. Phys. 1891, 99–116.
T. Soujanya, R. W. Fessenden, and A. Samanta (1996). Role of nonfluorescent twisted intramolecular charge transfer state on the photophysical behavior of aminophthalimide dyes. J. Phys. Chem. 1009, 3507–3512.
See for example, A. Zweig, A. H. Maurer, and B. G. Roberts (1967). Oxidation, reduction, and electrochemiluminescence of donor-substituted polycyclic aromatic hydrocarbons. J. Org. Chem. 32(5), 1322–1329 and references cited therein.
Y. Q. Gao and R. A. Marcus (2002). Theoretical investigation of the directional electron transfer in 4-aminonaphthalimide compounds. J. Phys. Chem. A 10610, 1956–1960.
G. Saroja and A. Samanta (1994). Steady state and time-resolved studies on the redox behavior of 1,8-naphthalimide in the excited state. J. Photochem. Photobiol. A Chem. 841, 19–26.
(a) D. Yuan and R. G. Brown (1997). Enhanced nonradiative decay in aqueous solutions of aminonaphthalimide derivatives via water-cluster formation. J. Phys. Chem. A 10119, 3461–3466. (b) M. S. Alexiou, V. Tychopoulos, S. Ghorbanian, J.H. P. Tyman, R. G. Brown, and P. I. Brittain (1990). The UV–Visible absorption and fluorescence of some substituted 1,8-naphthalimides and naphthalic anhydrides. J. Chem. Soc. Perkin Trans. 25, 837–842.
J. R. Lakowicz (1999). Principles of Fluorescence Spectroscopy, 2nd ed, Kluwer Academic/Plenum Publishers, New York.
(a) A. Pardo, E. Martin, J.M. L. Poyato, J. J. Camacho, M. F. Brana, and J. M. Castellano (1987). Synthesis and photophysical properties of some N-substituted-1,8-naphthalimides. J. Photochem. Photobilol. A Chem. 411, 69–78. (b) A. Pardo, E. Martin, J.M. L. Poyato, J. J. Camacho, J. M. Guerra, R. Weigand, M. F. Brana, and J. M. Castellano (1989). N-substituted 1,8-naphthalimide derivatives as high efficiency laser dyes. J. Photochem. Photobilol. A Chem. 48(2–3), 259–263. (c) A. Pardo, J.M. L. Poyato, E. Martin, J. J. Camacho, and D. Rayman (1990). Double exponential fluorescence decay in the protonation equilibrium of 4-methoxy-N-[2-(1-pyrrolidin)ethyl]-1,8-naphthalimide. J. Lumin. 466, 381–385. (d) A. P. de Silva, H.Q. N. Gunaratne, T. Gunnlaugsson, and P. L. M. Lynch (1996). Molecular photoionic switches with an internal reference channel for fluorescent pH sensing applications. New J. Chem. 20(7–8), 871–880.
(a) D. Noukakis, and P. Suppan (1991). Photophysics of aminophthalimides in solution. 1. Steady-state spectroscopy. J. Lumin. 476, 285–295. (b) E. Laitinen, K. Salonen, and T. O. Harju (1996). Solvation dynamics study of 4-amino-$N$-methyl-phthalimide in n-alcohol solutions. J. Chem. Phys. 10416, 6138–6148. (c) T. O. Harju, A. H. Huizer, and C. A. G. O. Varma (1995). Nonexponential salvation dynamics of electronically excited 4-aminophthalimide in n-alcohols. Chem. Phys. 200, 215. (d) S. Das, A. Datta, and K. Bhattacharyya (1997). Deuterium isotope effect on 4-aminophthalimide in neat water and reverse micelles. J. Phys. Chem. A101(18), 3299–3304. (e) H. Langhals (1991). A simple, quick, and precise procedure for the determination of water in organic-solvents.Anal. Lett.23(12), 2243–2258.
T. Okada, T. Saito, T. Mataga, Y. Sakata, and S. Misumi (1977). Bull. Chem. Soc. Jpn. 50, 331 and references cited therein.
J. W. Verhoeven, T. Schere, and R. J. Willemse (1993). Solvent effects on the structure of fluorescent exciplexes in rigidly-bridged, flexibly-bridged, and non-bridged donor-acceptor systems. Pure Appl. Chem. 658, 1717–1722.
(a) R. W. Kaplan, A. M. Napper, D. H. Waldeck, and M. B. Zimmt (2000). Solvent mediated coupling across 1 nm: Not a π-bond in sight. J. Am. Chem. Soc. 12248, 12039–12040. (b) H. Han and M. B. Zimmt (1998). Solvent-mediated electron transfer: Correlation between coupling magnitude and solvent vertical electron affinity. J. Am. Chem. Soc. 12031, 8001–8002.
R. S. Drago (1977). Physical Methods in Chemistry, Saunders College Publishing, Philadelphia.
(a) M. M. Martin, P. Plaza, N. Dai Hung, Y. H. Meyer, J. Bourson, and B. Valeur (1993). Photoejection of cations from complexes with a crown-ether-linked merocyanine evidenced by ultrafast spectroscopy. Chem. Phys. Lett. 2025, 425–430. (b) M. M. Martin, P. Plaza, Y. H. Meyer, L. Begin, J. Bourson, and B. Valeur (1994). A new concept of photogeneration of cations: Evidence for photoejection of Ca2+ and Li+ from complexes with a crown-ether-linked merocyanine by picosecond spectroscopy. J. Fluorescence 44, 271–275. (c) M. M. Martin, P. Plaza, Y. H. Meyer, F. Badaoui, J. Bourson, J. P. Lefebvre, and B. Valeur (1996). Steady-state and picosecond spectroscopy of Li+ or Ca2+ complexes with a crowned merocyanine. Reversible photorelease of cations. J. Phys. Chem. 10017, 6879–6888. (d) B. Valeur and I. Leray (2000). Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 205, 3–40.
(a) J. -F. Letard, R. Lapouyade, and W. Rettig (1993). Synthesis and photophysical study of 4-(N-monoaza-15-crown-5)stilbenes forming TICT states and their complexation with cations. Pure Appl. Cham. 658, 1705–1712. (b) P. Duman, G. Jonasauskas, F. Dupuy, P. Pee, C. Rulliere, J. -F. Letard, and R. Lapouyade (1994). Picosecond dynamics of cation-macrocycle interactions in the excited state of an intrinsic fluorescence probe: The calcium complex of 4-(N-monoaza-15-crown-5)-4′-phenylstilbene. J. Phys. Chem. 9841, 10391–10396. (c) R. Mathevet, G. Jonasauskas, C. Rulliere, J.-F. Letard, and R. Lapouyade (1995). Picosecond transient absorption as monitor of the stepwise cation-macrocycle decoordination in the excited singlet state of 4-(N-monoaza-15-crown-5)-4′-cyanostilbene. J. Phys. Chem. 9943, 15709–15713.
(a) A. Weller (1968). Pure Appl. Chem. 16, 118. (b) D. Rehm and A. Weller (1970). Isr. J. Chem. 8, 259.
H. Siegerman (1975). In N. L. Weinberg (Ed.), Techniques of Chemistry, Wiley, New York, Vol. V, Part II, p. 803.
See for example; (a) M. A. Fox and M. Chanon, (Eds.) (1998). Photoinduced Electron Transfer, Elsevier, New York, Parts A–D. (b) A. Weller, H. Staerk, and R. Trichel (1984). Faraday Discuss. Chem. Soc. 79, 271. (c) D. Gust and T. A. Moore (1991). In Photoinduced Electron Transfer J. Mattey, (Ed.), Topics in Current Chemistry 159, Vol. 3, Springer-Verlag, New York, p. 105. (d) J. W. Verhoeven (1990). Electron transport via saturated hydrocarbon bridges-exciplex emission from flexible rigid and semiflexible bichromophores. Pure Appl. Chem. 62, 1585. (e) K. D. Jordan and M. N. Paddon-Row (1992). Analysis of the interactions responsible for long-range through-bond-mediated electronic coupling between remote chromophores attached to rigid polynorbornyl bridges. Chem. Rev. 923, 395–410. (f) M. R. Wasielewski (1992). Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 923, 435–461. (g) G. J. Kavarnos (1993) Fundamentals of Photoinduced Electron Transfer, VCH, New York. (h) S. Speiser (1996). Photophysics and mechanisms of intramolecular electronic Energy transfer in bichromophoric molecular systems: Solution and supersonic jet studies. Chem. Rev. 966, 1953–1976. (i) J. M. Warman, S. A. Jonker, W. Schuddeboom, M. P. de Haas, M. N. Paddon-Row, J. W. Verhoeven, and K. A. Zachariasse (1993). Straight, bent and twisted intramolecular charge separated states as seen by time-resolved microwave conductivity (TRMC). Pure Appl. Chem. 658, 1723–1728. (j) A. Napper, I. Read, D. H. Waldeck, N. J. Head, A. M. Oliver, and M. N. Paddon-Row (2000). An unequivocal demonstration of the importance of nonbonded contacts in the electronic coupling between electron donor and acceptor units of donor-bridge-acceptor molecules. J. Am. Chem. Soc. 12221, 5220–5221. (k) I. Read, A. Napper, M. B. Zimmt, and D. H. Waldeck (2000). Electron transfer in aromatic solvents: The importance of quadrupolar interactions. J. Phys. Chem. A 104(41), 9385–9394. (l) I. Read, A. Napper, R. Kaplan, M. B. Zimmt, and D. H. Waldeck (1999). Solvent-mediated electronic coupling: The role of solvent placement. J. Am. Chem. Soc. 12147, 10976–10986. (m) R. J. Cave, M. D. Newton, K. Kumar, and M. B. Zimmt (1995). Theoretical study of solvent effects on the electronic coupling matrix element in rigidly linked donor–acceptor systems. J. Phys. Chem. 9949, 17501–17504.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badugu, R. Fluorescence Sensor Design for Transition Metal Ions: The Role of the PIET Interaction Efficiency. J Fluoresc 15, 71–83 (2005). https://doi.org/10.1007/s10895-005-0215-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10895-005-0215-9