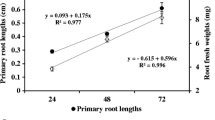
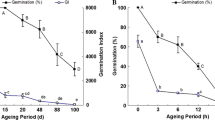
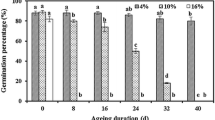
Abstract
The relationships between changes in energy metabolism and the antioxidant defense system in the weed species Ipomoea triloba L. during seed germination and early seedling growth were investigated. The effects of some common allelochemicals on these parameters also were studied. Respiratory activity and the activities of alcohol dehydrogenase, superoxide dismutase, catalase, guaicol peroxidase, ascorbate peroxidase, glutathione reductase, and lipoxygenase were measured. Mitochondrial oxidative phosphorylation resumed shortly after the seed imbibition period, as indicated by considerable KCN-sensitive respiratory activity in embryos of I. triloba. The occurrence of superoxide dismutase, catalase, guaicol peroxidase, and lipoxygenase activities in the embryos, along with significant KCN-insensitive respiration, suggest that production of reactive oxygen species (ROS) is initiated as soon as mitochondrial respiration is resumed. All assayed antioxidant enzymes were present in the embryos except ascorbate peroxidase, which appeared only in primary roots. The activities of antioxidant enzymes increased after completion of germination, especially in primary roots. Superoxide dismutase, catalase, and guaicol peroxidase probably were the crucial enzymes involved in the neutralization of ROS, since they had higher levels of activity compared with other enzymes, such as ascorbate peroxidase and glutathione reductase. When seeds were grown in the presence of α-pinene, coumarin, quercetin, and ferulic acid, there was an additional increase in activities of antioxidant enzymes, as well as increases in lipoxygenase activity and KCN-insensitive respiration, suggesting a further increase in ROS generation. The antioxidant defense system of I. triloba was not effective in preventing lipid peroxidation caused by α-pinene. The data indicate that during seed germination and initial growth of I. triloba, a period when antioxidant enzyme activity increases to counteract the harmful ROS effects produced during mitochondrial metabolism resumption, the presence of allelochemicals, which cause further oxidative stress, may leave the seeds/seedlings more vulnerable to cellular dysfunction and cell death.




Similar content being viewed by others
References
Abenavoli, M. R., Cacco, G., Sorgonà, A., Marabottini, R., Paolacci, A. R., Ciaffi, M., and Badiani, M. 2006. The inhibitory effect of coumarin on the germination of durum wheat (Triticum turgidum ssp. durum, CV. SIMETO) seeds. J. Chem. Ecol. 32:489–506.
Abrahim, D., Francisquine, A. C., Pergo, E. M., Bracht, A., and Ishii-Iwamoto, E. L. 2003a. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol. Biochem. 41:986–991.
Abrahim, D., Takahashi, L., Kelmer-Bracht, A. M., and Ishii-Iwamoto, E. L. 2003b. Effects of phenolic acids and monoterpenes on the mitochondrial respiration of soybean hypocotyls axes. Allelopathy J. 11:21–30.
Aebi, H. 1984. Catalase in vitro. Method. Enzymol. 105:121–126.
Almagro, L., Gómez-Ros, L. V., Belchi-Navarro, S., Bru, R., Ros-Barceló, A., and Pedreño, M. A. 2009. Class III peroxidases in plant defence reactions. J. Exp. Bot. 60:377–390.
Axelrod, B., Cheesbrough, T. M., and Zimmer, S. 1981. Lipoxygenase from soyabeans. Method. Enzymol. 71:441–451.
Bailly, C., Benamar, A., Corbineau, F., and Côme, D. 1996. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plantarum 97:104–110.
Bailly, C., Audigier, C., Ladonne, F., Wagner, M. H., Coste, F., Corbineau, F., and Côme, D. 2001. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot. 52:701–708.
Bailly, C., Bogatek-Leszczynska, R., Côme, D., and Corbineau, F. 2002. Changes in activity of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 12:47–55.
Bailly, C., El-Maarouf-Bouteau, H., and Corbineau, F. 2008. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C. R. Biologies 331:806–814.
Bais, H. P., Vepachedu, R., Gilroy, S., Callaway, R. M, and Vivanco, J. M. 2003. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:2377–1380.
Baker, C. J., and Orlandi, E. W. 1995. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33:299–321.
Batish, D. R., Setia, N., Singh. H. P., and Kohli, R. K. 2004. Phytotoxicity of Lemon-scented Eucalypt oil and its potential use as bioherbicide. Crop Prot. 23:1209–1214.
Batish, D. R., Singh, H. P., Setia, N., Kaur, S., and Kohli, R. K. 2006. 2-benzoxazolinone (BOA) induced oxidative stress, lipid peroxidation and changes in some antioxidant enzyme activities in mung bean (Phaseolus aureus). Plant Physiol. Biochem. 44:819–827.
Batish, D. R., Lavanya, K., Singh, H. P., and Kohli, R. K. 2007. Phenolic allelochemicals released by Chenopodium murale affect the growth nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 51:119–128.
Boyd, N., and Acker, R. V. 2004. Seed germination of common weed species as affected by oxygen concentration, light and osmotic potential. Weed Sci. 52:589–596.
Blokhin, A. O., Virolainen, E., and Fagerstedt, K. V. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Botany 91:179–194.
Chiapuasis, G., Sanchez, A. M., Reigosa, M. J., González, L., and Pellissier, F. 1997. Do germination indices adequately reflect allelochemical effects on the germination process?. J. Chem. Ecol. 23:203–209.
Chon, S. U., and Kim, Y. M. 2004. Herbicidal potential and quantification of suspected allelochemicals from four grass crop extracts. J. Agro. Crop Sci. 190:145–150.
Clifton, R., Lister, R., Parker, K. L., Sappl, P. G., Elhafez, D., Millar, A. H., Day, D. A., and Whelan, J. 2005. Stress-induced co-expression of alternative respiratory chain components in Arabidopsis thaliana. Plant Mol. Biol. 58:193–212.
Cruz-Ortega, R., Ayala-Cordero, G., and Anaya, A. L. 2002. Allelochemical stress produced by aqueous leachate of Callicarpa acuminate: effects on roots of bean, maize, and tomato. Physiol. Plantarum 116:20–27.
De Tullio, M. C., and Arrigoni, O. 2003. The ascorbic acid system in seeds: to protect and to serve. Seed Sci. Res. 13:249–260.
Dudai, N., Poljakoff-Mayber, A., Mayer, A. M., Putievsky, E., and Lerner, H. R. 1999. Essential oils as allelochemical and their potential use as bioherbicides. J. Chem. Ecol. 25:1079–1089.
Duke, S. O., Dayan, F. E., Romagni, J. G., and Rimando, A. M. 2000. Natural products as sources of herbicides: current status and future trends. Weed Res. 40:99–111.
Einhellig, F. A. 1995. Mechanism of action of allelochemicals in allelopathy, pp 96–116, in Inderjit, K. M. M. Dakshini and F. A. Einhellig (eds.). Allelopathy. Organims, processes, and applications. ACS Symposium Series 582, Washington DC, USA.
Estabrook, R. W. 1967. Mitochondrial respiratory control and polarographic measurements of ADP/O ratio. Method. Enzymol. 10:41–47.
Feussner, I., Balkenhol, T. J., Porzel, A., and Wasternack, C. 1997. Structural elucidation of oxygenated storage lipids in cucumber cotyledons. Implications of lipid body lipoxygenase in lipid mobilization during germination. J. Biol. Chem. 272:21635–21641.
Foyer, C. H., and Halliwell, B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 133:21–25.
Foyer, C. H., Lelandais, M., and Knert, K. J. 1994. Photooxidative stress in plants. Physiol. Plantarum. 92:696–717.
Giannopolitis, C. N., and Ries, S. K. 1977. Superoxide dismutases, I: occurrence in higher plants. Plant Physiol. 59:309–314.
Gidrol, X., Lin, W. S., Dégousée, N., Yip, S. F., and Kush, A. 1994. Accumulation of reactive oxygen species and oxidation of cytokinin in germination soybean seeds. Eur. J. Biochem. 224:21–28.
Golisz, A., Sugano, M., and Fuji, Y. 2008. Microarray expression profile of Arabidopsis thaliana L. in response to allelochemcials identified in buckwheat. J. Exp. Bot. 59:3099–3109.
Goodman, B. A., Mcphail, D. B., and Linehan, D. J. 1986. Oxygen-induced free radical in wheat roots. Free Radic. Res. Commun. 2:173–178.
Heath, R. L., and Packer, L. 1968. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acids peroxidation. Arch. Biochem. Biophys. 125:189–198.
Hildebrand, D. F. 1989. Lipoxygenases. Physiol. Plantarum 76:249–253.
Holm, L., Doll, J., Holm, E., Pancho, J., and Herberger, J. 1997. World weeds, natural histories and distribution, in J. WILEY and S. INC., New York, USA.
Holtman, W. L., Van Duijn, G., Sedee, N. J. A., and Douma, A. C. 1996. Differential expression of lipoxygenase isozymes in embryos of germinationg barley. Plant Physiol. 111:569–576.
Inderjit, and Duke, S. O. 2003. Ecophysiological aspects of allelopathy. Planta. 217:529–539.
Ishii-Iwamoto, E. L., Abrahim, D., Sert, M. A., Bonato, C. M., Kelmer-Bracht, A. M., and Bracht, A. 2006. Mitochondria as a site of allelochemical action, pp. 267–284, in M. J. Reigosa, N. Pedrol and L. González (eds.) Allelopathy: A Physiological Process with Ecological Implications. (Org) Springer Science, Netherlands.
Kern, K. A., Pergo, E. M., Kagami, F. L., Arraes, L. S., Sert, M. A., and Ishii-Iwamoto, E. L. 2009. The phytotoxic effect of exogenous ethanol on Euphorbia heterophylla L. Plant Physiol. Biochem. 47:1095–1101.
Kohli, R. K., Batish, D. R., and Singh, H. P. 2006. Allelopathic interactions in agroecosystems, pp. 465-493, in M. J. Reigosa, N. Pedrol and L. González (eds.). Allelopathy: A Physiological Process with Ecological Implications. Springer, Netherlands.
Kruse, N. D., Vidal, R. A., Dalmarz, C., Trezzi, M. M., and Siqueira, I. 2006. Estresse oxidativo em girasol (Helianthus annuus) indica sinergismo para a mistura dos herbicidas metribuzin e clomazone. Planta Daninha 24:379–390.
Kwak, J. M., Nguyen, V., and Schoeder, J. I. 2006. The role of reactive oxidative species in hormonal responses. Plant Physiol. 141:323–329.
Labouriau, L. G., and Osborn, J. H. 1984. Temperature dependence of the germination of tomato seeds. J. Thermal Biol. 9:285–294.
Larkin, P. J. 1987. Calmodulin levels are not responsible for aluminum tolerance in wheat. Aust. J. Plant Physiol. 14:377–387.
Lee, C. Y. 1982. Alcohol dehydrogenase from Drosophila melanogaster. Method Enzymol. 89:445–450.
Lowry, O., Rosebrough, N. J., and Farr, A. L. 1951. Protein measurements with the folin phenol. J. Biol. Chem. 193:265–275.
Macías, F. A. 1995. Allelopathy in the search for natural herbicide models, pp. 310–327, in Inderjit, K. M. M. Dakshini and F. A. Einhellig (eds.). Allelopathy. Organims, Processes, and Applications. ACS Symposium Series 582, Washington DC, USA.
Maxweel, D. P., Wang, Y., and McIntosh, L. 1999. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl Acad. Sci USA 96:8271–8276.
McDonald, M. B. 1999. Seed deterioration: physiology, repair and assessment. Seed Sci. Technol. 27:177–237.
Mittler, R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410.
Müsel, G., Schindler, T., Bergfeld, R., Ruel, K., Jaquet, G., Lapieree, C., Speth, V., and Schopepfer, P. 1997. Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological probes. Planta. 201:146–159.
Nakano, Y., and Asada, K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867–880.
Navrot, N., Rouhier, N., Gelhaye, E., and Jacquot, J. P. 2007. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plantarum, 129:185–195.
Noctor, G., and Foyer, C. H. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Phys. 49:249–279.
Oracz, K., El-Maarouf-Bouteau, H., Kranner, I., Bogatek, R., Corbineau, F., and Bailly, C. 2009. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol. 150:496–505.
Passardi, F., Cosio, C., Penel, C., and Dunand, C. 2005. Peroxidases have more function than a Swiss army knife. Plant Cell Rep. 24:255–265.
Pergo, E. M., Abrahim, D., Silva, P. C. S., Kern, K. A., Silva, L. J., Voll, E., and Ishii-Iwamoto, E. L. 2008. Bidens pilosa L. exhibits high sensitivity to coumarin in comparison with three other weed species. J. Chem. Ecol. 34:499–507.
Porta, H., and Rocha-Sosa, M. 2002. Plant Lipoxygenases. Physiological and Molecular Features. Plant Physiol. 130:15–21.
Puntarullo, S., Galleano, M., Sanchez, R. A., and Boveris, A. 1988. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol. 86:626–630.
Pütter, J. 1974. Peroxidases, pp. 685, in H. U. Bergmeryer (ed.). Methods of Enzymatic Analysis. Verlag Chemie, Weinheim, Academic Press Inc, New York, USA.
Qian, H., Xu, X., Chen, W., Jiang, H., Yuanxiang, J., Liu, W., and Fu, Z. 2009. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Clorella vulgaris. Chemosphere 75:368–375.
Quiroga, M., Guerrero, C., Botella, M. A., Barceló, A., Amaya, I., Medina, M. I., Alonso, F. J., Milrad De Forchetti, S., Tigier, H., and Valpuesta, V. 2000. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 122:1119–1128.
Reigosa, M. J., Souto, X. C., and González, L. 1999. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 28:83–88.
Rodriguez-Conception, M., Gòmez, M. D., and Beltran, J. P. 1995. Repression of the pea lipoxygenase gene lox g is associated with carpel development. Plant Mol. Biol. 27:887–899.
Romero-Romero, T., Anaya, A. L., and Cruz-Ortega, R. 2002. Screening for effects of phytochemical variability on cytoplasmic protein synthesis pattern of crop plants. J. Chem. Ecol. 28:617–629.
Rudrappa, T., Bonsall, J., Gallagher, J. L., Seliskar, D. M., and Bais, H. P. 2007. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol. 33:1898–1918.
Sert, M. A., Ferraresi, M. L. L., Bernardelli, Y. R., Kelmer-Bracht, A. M., Bracht, A., and Ihiii-Iwamoto, E. L. 1998. Effects of ferulic acid on L-malate oxidation in isolated soybean mitochondria. Biol. Plantarum. 40:345–350.
Siedow, J. N., and Girvin, M. E. 1980. Alternative respiratory pathway. Plant Physiol. 65:669–674.
Siedow, J. N. and Moore, A. L. 1991. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitocochondria. Biochim. Biophys. Acta 1059:121–140.
Singh, H. P., Batish, D. R., Kaur, S., Arora, K., and Kohli, R. K. 2006. Alfa-pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 98:1261–1269.
Smith, I. K. 1985. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 79:1044–1047.
Staniek, K., and Nohl, H. 2000. Are mitochondria a permanent source of reactive oxygen species? Biochim. Biophys. Acta. 1460:268–275.
Stoller, E. W., and Weber, E. J. 1970. Lipid constituents of some common weed seeds. J. Agr. Food Chem. 18:361–363.
Takahashi, L. T., Sert, M. A., Kelmer-Bracht, A. M., Bracht, A., and Ishii-Iwamoto, E. L. 1998. Effects of rutin and quercetin on mitochondrial metabolism and on ATP levels in germination tissues of Glycine max. Plant Physiol. Biochem. 36:495–501.
Thaler, J. S. 1999. Induced resistance in agricultural crops: effects of jasmonic acid on herbivory and yield in tomato plants. Envirom. Entomol. 28:30–37.
Tranbarger, T. J., Franceschi, V. R., Hildebrand, D. F., and Grimes, H. D. 1991. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3:973–987.
Weir, T. L., Sang-Wood, P., and Vivanco, J. M. 2004. Biochemical and physiological mechanism mediated by allelochemicals. Curr. Opin. Plant Biol. 7:472–479.
Wojtyla, L., Garnczarska, M., Zalewski, T., Bednarski, W., Ratajczak, L., and Jurga, S. 2006. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. J. Plant Physiol. 163:1207–1220.
Vaughn, S. F., and Spencer, G. F. 1993. Volatile monoterpenes as potential parent structures for new herbicides. Weed Sci. 41:114–119.
Acknowledgements
This work was supported by grants from the Fundação Araucária do Estado do Paraná and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Érica Marusa Pergo holds a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pergo, É.M., Ishii-Iwamoto, E.L. Changes in Energy Metabolism and Antioxidant Defense Systems During Seed Germination of the Weed Species Ipomoea triloba L. and the Responses to Allelochemicals. J Chem Ecol 37, 500–513 (2011). https://doi.org/10.1007/s10886-011-9945-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9945-0