Abstract
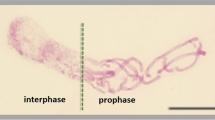
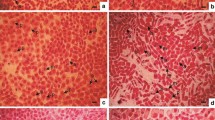
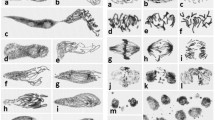
Several benzoic and cinnamic acid derivatives were identified from cucumber root exudates. The effects of these phenylcarboxylic acids on root growth and cell cycle progression were examined in germinated seeds of cucumber. All 12 phenylcarboxylic acids (0.25 mM) tested significantly inhibited cucumber radicle growth, and cinnamic acid exerted a dose-dependent inhibitory effect. At 6 h after exposure to the acids, transcript levels of the cell cycle-related genes, including two cyclin-dependent kinases (CDKs) and four cyclins were reduced. Among them, transcript of CycB, a marker gene for mitosis showed a remarkable reduction. The temporal analysis showed that expression of mitotic genes (CDKB, CycA, and CycB) were reduced throughout the experiment, while the reduction of the other genes (CDKA, CycD3;1, and CycD3;2) were observed only at earlier time points. At 48 h after treatment with benzoic and cinnamic acids, an enhancement of endoreduplication was observed. Further time course analysis indicated that endoreduplication started as early as 6 h after exposure to cinnamic acid. These results provide evidence that exposure to benzoic and cinnamic acids can induce rapid and dramatic down-regulation of cell cycle-related genes, thus leading to root growth inhibition. Meanwhile, the block of mitosis caused by phenylcarboxylic acids also induced an increased level of endoreduplication.




Similar content being viewed by others
References
An, M., Pratley, J. E., and Haig, T. 2001a. Phytotoxicity of vulpia residues: III. Biological activity of identified alleochemicals from Vulpia myuros. J. Chem. Ecol. 27, 383–394.
An, M., Pratley, J. E., and Haig, T. 2001b. Phytotoxicity of vulpia residues: IV. Dynamics of allelochemicals during decomposition of vulpia residues and their corresponding phytotoxicity. J. Chem. Ecol. 27, 395–409.
Asao, T., Pramanik, M. H. R., Tomita, K., Oba, Y., Ota, K., Hosoki, T., and Matsui, Y. 1999. Influences of phenolics isolated from the nutrient solution nourishing growing cucumber (Cucumis sativus L.) plants on fruit yield. J. Jpn. Soc. Hortic. Sci. 68: 847–853.
Barow, M. and Meister, A. 2003. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Environ. 26: 571–584.
Batish, D. R., Singh, H. P., Kaur, S., Kohli, R. K., and Yadav, S. S. 2008. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 165: 297–305.
Beemster, G. T. S., De Vusser, K., De Tavernier, E., De Bock, K., and Inzé, D. 2002. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 129:854–864.
Beemster, G. T. S., Fiorani, F., and Inzé, D. 2003. Cell cycle: the key to plant growth control? Trends Plant Sci. 8: 154–158.
Beemster, G. T. S., De Veylder, L., Vercruysse, S., West, G., Rombaut, D., Hummelen, P. V., Galichet, A., Gruissem, W., Inzé, D., and Vuylsteke, M. 2005. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol. 138: 734–743.
Blum, U. 1996. Allelopathic interactions involving phenolic acids. J. Nematol. 28:259–267.
Boudolf, V., Vlieghe, K., Beemster, G. T. S., Magyar, Z., Acosta, J. A. T., Maes, S., Van Der Schueren, E., Inzé, D., and De Veyldera, L. 2004. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 16: 2683–2692.
Burgos, N. R., Talbert, R. E., Kim, K. S., and Kuk, Y. I. 2004. Growth inhibition and root ultrastructure of cucumber seedlings exposed to allelochemicals from rye (Secale cereale). J. Chem. Ecol. 30: 671–689.
Callaway, R. M. and Aschehoug, E. T. 2000. Invasive plant versus their new and old neighbors: a mechanism for exotic invasion. Science 290, 521–523.
Chon, S. U., Choi, S. K., Jung, S., Jang, H. G., Pyo, B. S., and Kim, S. M. 2002. Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of alfalfa and barnyard grass. Crop. Prot. 21:1077–1082.
Cockcroft, C. E., Den Boer, B. G. W., Healy, J. M. S., and Murray, J. A. H. 2000. Cyclin D control of growth rate in plants. Nature 405:575–579.
Cools, T. and De Veylder, L. 2009. DNA stress checkpoint control and plant development. Curr. Opin. Plant Biol. 12: 23–28.
Ding, J., Sun, Y., Xiao, C. L., Shi, K., Zhou, Y. H., and Yu, J. Q. 2007. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 58:3765–3773.
Dos Santos, W. D., Ferrarese, M. L. L., Nakamura, C. V., Mourão, K. S. M., Mangolin, C. A., and Ferrarese-Filho, O. 2008. Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J. Chem. Ecol. 34:1230–1241.
Ferreira, P., Hemerly, A., De Almeida Engler, J., Bergounioux, C., Burssens, S., Van Montagu, M., Engler, G., and Inzé, D. 1994. Three discrete classes of Arabidopsis cyclins are expressed during different intervals of the cell cycle. Proc. Natl. Acad. Sci. USA. 91: 11313–11317.
Fusconi, A., Repetto, O., Bona, E., Massa, N., Gallo, C., Dumas-Gaudot, E., and Berta, G. 2006. Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ. Exp. Bot. 58:253–260.
Galbraith, D. W., Harkins, K. R., Maddox, J. M., Ayres, N. M., Sharma, D. P., and Firoozabad, Y. E. 1983. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051.
Galbraith, D. W., Harkins, K. R., and Knapp, S. 1991. Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96:985–989.
Gilissen, L. J. W., Van Staveren, M. J., Creemers-Molenaar, J., and Verhoeven, H. A. 1993. Development of polysomaty in seedlings and plants of Cucumis sativus L. Plant Sci. 91: 171–179.
Golisz, A., Sugano, M., and Fujii, Y. 2008. Microarray expression profiling of Arabidopsis thaliana L. in response to allelochemicals identified in buckwheat. J. Exp. Bot. 59:3099–3109.
Gonzalez, V. M., Kazimir, J., Nimbal, C., Weston, L. A., and Cheniae, G. M. 1997. Inhibition of a photosystem II electron transfer reaction by the natural product sorgoleone. J. Agric. Food Chem. 45:1415–1421.
Greilhuber, J. 2008. Cytochemistry and C-values: the less-well-known world of nuclear DNA amounts. Ann. Bot. 101:791–804.
Greilhuber, J., Doležel, J., Lysák, M.A., and Bennett, M.D. 2005. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann. Bot. 95:255–260.
Hejl, A. M. and Koster, K. L. 2004. The allelochemical sorgoleone inhibits root H+-ATPase and water uptake. J. Chem. Ecol. 30:2181–2191.
Hejl, A. M., Einhellig, F. A., and Rasmussen, J. A. 1993. Effects of juglone on growth, photosynthesis, and respiration. J. Chem. Ecol.19:559–568.
Hemerly, A., Bergounioux, C., Van Montagu, M., Inzé, D., and Ferreira, P. 1992. Genes regulating the plant cell cycle: isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 89: 3295–3299.
Hemerly, A., De Almeida, E. J., Bergounioux, C., Van, M. M., Engler, G., Inzé, D., and Ferreira, P. 1995. Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 14: 3925–3936.
Hiradate, S., Morita, S., Furubayashi, A., Fujii, Y., and Harada, J. 2005.Plant growth inhibition by cis-cinnamoyl glucosides and cis-cinnamic acid. J. Chem. Ecol. 31:591–601.
Inderjit, S., and Duke, S. O. 2003. Ecophysiological aspects of allelopathy. Planta 217: 529–539.
Inzé, D. 2005. Green light for the cell cycle. EMBO J. 24:657–662.
Inzé, D. and De Veylder, L. 2006. Cell cycle regulation in plant development. Ann. Rev. Genet. 40: 77–105.
Iqbal, Z., Hiradate, S., Araya, H., and Fujii, Y. 2004. Plant growth inhibitory activity of Ophiopogon japonicus Ker-Gawler and role of phenolic acids and their analogues: a comparative study. Plant Growth Regul. 43: 245–250.
Jakoby, M. and Schnittger, A. 2004. Cell cycle and differentiation. Curr. Opin. Plant Biol. 7:661–669.
Joubès, J. and Chevalier, C. 2000. Endoreduplication in higher plants. Plant Mol. Biol. 43:735–745.
Livak, K. J. and Schmittgen, T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCT method. Methods 25: 402–408.
Magyar, Z., Mészáros, T., Miskolczi, P., Deák, M., Fehér, A., Brown, S., Kondorosi, E., Athanasiadis, A., Pongor, S., Bilgin, M., Bakó, L., Koncz, C., and Dudits, D. 1997. Cell cycle phase specificity of putative cyclin dependent kinase variants in synchronized alfalfa cells. Plant Cell 9: 223–235.
Menges, M., Hennig, L., Gruissem, W., and Murray, J. A. H. 2002. Cell cycle-regulated gene expression in Arabidopsis. J. Biol. Chem. 277: 41987–42002.
Menges, M., Samland, A. K., Planchais, S., and Murray, J. A. H. 2006. The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell 18:893–906.
Nishida, N., Tamotsu, S., Nagata, N., Saito, C., and Sakai, A. 2005. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 31:1187–1203.
Porceddu, A., Stals, H., Reichheld, J-P., Segers, G., De Veylder, L., De Pinho Barrôco, R., Casteels, P., Van Montagu, M., Inzé, D., and Mironov, V. 2001. A plant-specific cyclin-dependent kinase is involved in the control of G(2)/M progression in plants. J. Biol. Chem. 276: 36354–36360.
Reichheld, JP., Vernoux, T., Lardon, F., Van Montagu, M., and Inzé, D. 1999. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J. 17:647–656.
Repetto, O., Massa, N., Gianinazzi-Pearson, V., Dumas-Gaudot, E., and Berta, G. 2007. Cadmium effects on populations of root nuclei in two pea genotypes inoculated or not with the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 17:111–120.
Rice, E. L. 1984. Allelopathy. 2nd ed., Academic Press New York.
Rudrappa, T., Bonsall, J., Gallagher, J. L., Seliskar, D. M., and Bais, H. P. 2007. Root-secreted allelochemical in the noxious weed Phragmites Australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol. 33:1898–1918.
Rymen, B., Fiorani, F., Kartal, F., Vandepoele, K., Inzé, D., and Beemster, G. T. S. 2007. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 143:1429–1438.
Sánchez-Moreiras, A. M., De La Peña, T. C., and Reigosa, M. J. 2008. The natural compound benzoxazolin-2(3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry 69:2172–2179.
Schnittger, A., Schöbinger, U., Stierhof, Y. D., and Hülskamp, M. 2002. Ectopic B-type cyclin expression induces mitotic cycles in endoreduplicating Arabidopsis trichomes. Curr. Biol. 12:415–420.
Setter, T. L. and Flannigan, B. A. 2001. Water deficit inhibits cell division and expression of transcripts involved in cell proliferation and endoreduplication in maize endosperm. J. Exp. Bot. 52:1401–1408.
Singh, H. P., Batish, D. R., and Kohli, R. K. 1999. Autotoxicity: concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 18:757–772.
Suzuki, K., Nishiuchi, T., Nakayama, Y., Ito, M., and Shinshi, H. 2006. Elicitor-induced down-regulation of cell cycle-related genes in tobacco cells. Plant Cell Environ. 29:183–191.
Tharayil, N., Bhowmik, P. C., and Xing, B. S. 2008. Bioavailability of allelochemicals as affected by companion compounds in soil matrices. J. Agr. Food. Chem. 56:3706-3713.
Weidenhamer, J. D., Hartnett, D. C., and Romeo, J. T. 1989. Density dependent phytotoxicity: distinguishing resource competition and allelopathic interference in plants. J. Appl. Ecol. 26: 613–624.
Weir, T. L., Park, S. W., and Vivanco, J. M. 2004. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol. 7: 472–479.
West, G., Inzé, D., and Beemster, G. T. S. 2004. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 135:1050–1058.
Ye, S. F., Yu, J. Q., Peng, Y. H., Zheng, J. H., and Zou, L. Y. 2004. Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 263: 143–150.
Ye, S. F., Zhou, Y. H., Sun, Y., Zou, L. Y., and Yu, J. Q. 2006. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 56: 255–262.
Yu, J.Q. and Matsui, Y. 1994. Phytotoxic substances in the root exudates of cucumber (Cucumis sativus L.). J. Chem. Ecol. 20: 21–31.
Yu, J.Q. and Matsui, Y. 1997. Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on uptake by cucumber seedlings. J. Chem. Ecol. 23: 817–827.
Yu, J. Q., Shou, S. Y., Qian, Y. R., and Hu, W. H. 2000. Autotoxic potential in cucurbit crops. Plant Soil 223: 147–151.
Yu, J. Q., Ye, S. F., Zhang, M. F., and Hu, W. H. 2003. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 31: 129–139.
Acknowledgement
This work was supported by the National Basic Research Program of China (2009CB11900), National Natural Science Foundation of China (40571083; 3050344; 30671428) and PhD Program of MOE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Gu, M., Xia, X. et al. Effects of Phenylcarboxylic Acids on Mitosis, Endoreduplication and Expression of Cell Cycle-Related Genes in Roots of Cucumber (Cucumis sativus L.). J Chem Ecol 35, 679–688 (2009). https://doi.org/10.1007/s10886-009-9642-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9642-4