Abstract
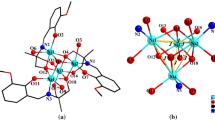
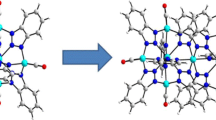
A novel [Ni4(µ3-O)4] twisted cubane complex, Ni4Cl2(NN)2(ONN)2(NNO)2(H2O)2·2THF, was prepared from a crude mixture of three bidentate ligands. A mixture of ethyl 1-(hydroxymethyl)-3-methyl-1H-pyrazole-5-carboxylate (HONN) and ethyl 1-(hydroxyl-methyl)-5-methyl-1H-pyrazole-3-carboxylate (NNOH) were added to ethyl 3-methyl-1H-pyrazole-5-carboxylate (NNH) then mixed to NiCl2·6H2O solution under ambient conditions. Reaction progress was monitored via infrared and ultraviolet–visible spectroscopies and energy-dispersive X-ray spectroscopy was used to analyze the product. Reaction yields for cluster synthesis were very good. Single crystal structure determination for the cluster indicates a novel [Ni4(µ3-O)4] twisted cubane structure with octahedral geometry around each of the Ni(II) centers in Ni4Cl2(NN)2(ONN)2(NNO)2(H2O)2·2THF. The lattice is stabilized by hydrogen bonding and H–\( \pi \) stacking. Hirshfeld surface analysis (HSA) corroborates the single crystal structure determination results. The cluster displays significant thermal stability under open atmosphere conditions; it decomposes in three steps at high temperature. The cluster demonstrates promising results as a catalyst; it promoted the complete oxidation of catechol to o-Quinone in under mild conditions.








Similar content being viewed by others
References
A. Martínez, J. Lorenzo, M. J. Prieto, M. Font-Bardia, X. Solans, F. X. Avilés, and V. Moreno (2007). Bioorg. Med. Chem. 15, 969.
B. Antonioli, D. J. Bray, J. K. Clegg, K. A. Jolliffe, K. Gloe, K. Gloe, and L. F. Lindoy (2007). Polyhedron 26, 673.
J. Maroszová, J. Moncol, Z. Padelková, R. Sillanpä, T. Lis, and M. Koman (2011). Cent. Eur. J. Chem. 9, 453.
T. C. Stamatatos, K. A. Abboud, W. Wernsdorfer, and G. Christou (2007). Angew. Chem. 119, 902.
F.-M. Wang, C.-S. Lu, Y.-Z. Li, and Q.-J. Meng (2010). Acta Crystallogr. Sect. E E66, m594.
T. C. Stamatatos, A. K. Boudalis, K. V. Pringouri, C. P. Raptopoulou, A. Terzis, J. Wolowska, E. J. L. McInnes, and S. P. Perlepes (2007). Eur. J. Inorg. Chem. 2007, 5098.
C. Icsel, V. T. Yilmaz, F. Ari, E. Ulukaya, and W. T. A. Harrison (2013). Eur. J. Med. Chem. 60, 386.
A. Jabłonska-Wawrzycka, B. Barszcz, M. Zienkiewicz, M. Hodorowicz, J. Jezierska, K. Stadnicka, Ł. Lechowicz, and W. K. Spectrochim (2014). Acta A: mol. Biomol. Spectrosc. 129, 632.
M. Zienkiewicz, A. Jabłonska-Wawrzycka, J. Szlachetko, Y. Kayser, K. Stadnicka, W. Sawka Dobrowolska, J. Jezierska, and B. Barszcz (2014). J. Sá, Dalton Trans 43, 8599.
M. Zienkiewicz, J. Szlachetko, C. Lothschütz, M. Hodorowicz, A. Wawrzycka, J. Sá, and B. Barszcz (2013). Dalton Trans. 42, 7761.
M. El Kodadi, F. Malek, R. Touzani, and A. Ramdani (2008). Catal. Commun. 9, 966.
E. Kim, H. Y. Woo, S. Kim, H. Lee, D. Kim, and H. Lee (2012). Polyhedron 42, 135.
A. Otero, J. Fernández-Baeza, A. Lara-Sánchez, and L. F. Sánchez-Barba (2013). Coord. Chem. Rev. 257, 1806.
F. Xue, J. Zhao, and T. S. A. Hor (2013). Dalton Trans. 42, 5150.
M. Yang, W. J. Park, K. B. Yoon, J. H. Jeong, and H. Lee (2011). Inorg. Chem. Commun. 14, 189.
N. Boussalah, R. Touzani, I. Bouabdallah, S. El Kadiri, and S. Ghalem (2009). J. Mol. Catal. A: Chem. 306, 113.
M. Scarpellini, J. Gätjens, O. J. Martin, J. W. Kampf, S. E. Sherman, and V. L. Pecoraro (2008). Inorg. Chem. 47, 3584.
M. Scarpellini, A. J. Wu, J. W. Kampf, and V. L. Pecoraro (2005). Inorg. Chem. 44, 5001.
M. D. Kärkäs, O. Verho, E. V. Johnston, and B. Åkermark (2014). Chem. Rev. 114, 11863.
Y. Umena, K. Kawakami, J.-R. Shen, and N. Kamiya (2011). Nature 473, 55.
X.-B. Han, Y.-G. Li, Z.-M. Zhang, H.-Q. Tan, Y. Lu, E.-B. Wang (2015.) J. Am. Chem. Soc.137, 5486. b) A. K. Poulsen, A. Rompel, C. J. McKenzie, Angew (2005). Chem. Int. Ed. 44, 6916.
A. Titi, T. Shiga, H. Oshio, R. Touzani, B. Hammouti, M. Mouslim, and I. Warad (2020). J. Mol. Struc. 1199, 126995.
S. Mukhopadhyay, S. K. Mandal, S. Bhaduri, and W. H. Armstrong (2004). Chem. Rev. 104, 3981.
K. Wolff, D. J. Grimwood, J. J. McKinnon, D. Jayatilaka, and M. A. Spackman Crystal Explorer 2.1 (University of Western Australia, Perth, 2007).
G. M. Sheldrick (2008). Acta Cryst. 64, 112.
G. Huang, S. Hua, I. Po-Chun Liu, C. Chien, J. Kuo, G. Lee, and S. Peng (2012). C. R. Chimie 15, 159.
M. Jana, J. L. Priego, R. Jiménez-Aparicio, and T. K. Mondal (2014). Mondal. Spectrochimica Acta A 133, 714.
I. Warad, F. F. Awwadi, B. Abd Al-Ghani, A. Sawafta, N. Shivalingegowda, N. K. Lokanath, M. S. Mubarak, T. Ben Hadda, A. Zarrouk, F. Al-Rimawi, A. B. Odeh, and S. A. Barghouthi (2018). Ultrasonics Sonochem. 48, 1.
I. Warad, Y. Al-Demeri, M. Al-Nuri, S. Shahwan, M. Abdoh, S. Naveen, N. K. Lokanath, M. S. Mubarak, T. B. Hadda, and Y. N. Mabkhot (2017). J. Mol. Struct. 1142, 217.
F. A. Saleem, S. Musameh, A. Sawafta, P. Brandao, C. J. Tavares, S. Ferdov, A. Barakat, A. Al Ali, M. Al-Noaimi, and I. Warad (2017). Arab. J. Chem. 10, 845.
I. Warad and A. Barakat (2017). J. Mol. Struc. 1134, 17.
K. S. Joya, L. Sinatra, L. G. AbdulHalim, C. P. Joshi, M. N. Hedhili, O. M. Bakrb, and I. Hussain (2015). Nanoscale 112, 1.
B. K. Das and R. Chakrabarty (2011). J. Chem. Sci. 123, 163.
A. Zerrouki, R. Touzani, and S. El Kadiri (2011). Arab. J. Chem. 4, 459.
A. Mouadili, A. Zerrouki, L. Herrag, B. Hammouti, S. El Kadiri, and R. Touzani (2012). Res. Chem. Interm. 38, 2427.
H. Boulemche, B. Anak, A. Djedouani, R. Touzani, M. Francois, S. Fleutot, and F. Rabilloud (2019). Mol. Struc. 1178, 606.
Z. Bouanane, M. Bounekhel, M. Elkolli, F. Abrigach, M. Khoutoul, R. Boyaala, R. Touzani, and A. Hellal (2017). J. Mol. Struc. 1139, 238.
R. Modak, Y. Sikdar, S. Mandal, and S. Goswami (2013). Inorg. Chem. Commun. 09, 26.
S. Indira, G. Vinoth, M. Bharathi, S. Bharathi, A. K. Rahiman, and K. S. Bharathi (2019). Inorg. Chim. Acta 495, 118988.
M. N. Ahamad, F. Sama, M. N. Akhtar, Y.-C. Chen, M.-L. Tong, M. Ahmad, M. Shahid, S. Hussain, and K. Khan (2017). New J. Chem. 41, 14057.
Acknowledgements
Researchers Supporting Project number (RSP-2019/78), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10876_2020_1780_MOESM1_ESM.docx
Supplementary material 3 (DOCX 414 kb). Fig. 1S Computed (a) dnorm surface, (b) shape index, (c) curvedness structures, and (d) inside/outside two-dimensional fingerprint plots.
Rights and permissions
About this article
Cite this article
Titi, A., Oshio, H., Touzani, R. et al. Synthesis and XRD of Novel Ni4(µ3-O)4 Twist Cubane Cluster Using Three NNO Mixed Ligands: Hirshfeld, Spectral, Thermal and Oxidation Properties. J Clust Sci 32, 227–234 (2021). https://doi.org/10.1007/s10876-020-01780-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01780-0