Abstract
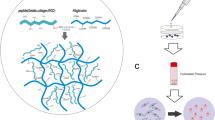
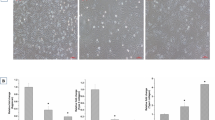
Strategies based on growth factor (GF) delivery have attracted considerable attention in tissue engineering applications. Among different GFs, transforming growth factor beta 1 (TGF-β1) is considered to be a potent factor for inducing chondrogenesis. In the present study, an expression cassette encoding the TGF-β1 protein was prepared and transfected into the SP2/0-Ag14 cell line. The confocal microscopy of the transfected cells was performed to confirm the correct transfection process. The expression and in vitro release kinetics of the recombinant TGF-β1 were assessed by western blot analysis and ELISA, respectively. Moreover, the biological activity of the expressed protein was compared with that of a commercially available product. The chondrogenic effects of the sustained release of the recombinant TGF-β1 in an in vitro co-culture system were evaluated using a migration assay and real-time PCR. Results of confocal microscopy confirmed the successful transfection of the vector-encoding TGF-β1 protein into the SP2/0-Ag14 cells. The bioactivity of the produced protein was in the range of the commercial product. The sustained release of the TGF-β1 protein via SP2/0-Ag14 cells encapsulated in hydrogels encouraged the migration of adipose-derived MSCs. In addition, the expression analysis of chondrogenesis-related genes revealed that the pretreatment of encapsulated Ad-MSCs cells in alginate sulfate hydrogels through their exposure to the sustained release of TGF-β1 is an efficient approach before transplantation of cells into the body.









Similar content being viewed by others
References
Moradi L, Vasei M, Dehghan MM, Majidi M, Mohajeri SF, Bonakdar S. Regeneration of meniscus tissue using adipose mesenchymal stem cells-chondrocytes co-culture on a hybrid scaffold: in vivo study. Biomaterials. 2017;126:18–30.
Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res. 2012;347(3):613–27.
Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31(5):706–21.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920.
Moreira-Teixeira LS, Georgi N, Leijten J, Wu L, Karperien M. Cartilage tissue engineering. In: Camacho-Hübner C, Nilsson O, Sävendahl l, (eds.) Cartilage and bone development and its disorders. Karger Publishers. 2011. p. 102–15.
Lindahl A, Brittiberg M, Gibbs D, Dawson JI, Kanczler J, Black C. et al. Cartilage and bone regeneration. In: De Boer J, Van Blitterswijk C (eds) Tissue engineering. 2nd edn. Amsterdam, Netherland: Elsevier; 2015. p. 529–82.
Toh WS, Foldager CB, Pei M, Hui JH. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev Rep. 2014;10(5):686–96.
Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–6.
Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic differentiation of human embryonic stem cell–derived cells in arginine-glycine-aspartate–modified hydrogels. Tissue Eng. 2006;12(9):2695–706.
Yu D-A, Han J, Kim B-S. Stimulation of chondrogenic differentiation of mesenchymal stem cells. Int J Stem Cells. 2012;5(1):16 p.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses. 2008;71(6):900–8.
Farajollahi MM, Hamzehlou S, Mehdipour A, Samadikuchaksaraei A. Recombinant proteins: hopes for tissue engineering. BioImpacts. 2012;2(3):123
Poniatowski, ŁA, Wojdasiewicz P, Gasik, Szukiewicz D. Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators inflamm. 2015; 137823, https://doi.org/10.1155/2015/137823.
Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679–88.
Govinden R, Bhoola K. Genealogy, expression, and cellular function of transforming growth factor-β. Pharmacol Ther. 2003;98(2):257–65.
Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233:532–5.
Iwasa K, Reddi AH. Optimization of methods for articular cartilage surface tissue engineering: cell density and transforming growth factor beta are critical for self-assembly and lubricin secretion. Tissue Eng Part C: Methods. 2017;23(7):389–95.
Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G. et al. Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-β and is ameliorated by the alternative supplementation of latent TGF-β. Biomaterials. 2016;77:173–85.
Kim J, Lin B, Kim S, Choi B, Evseenko D, Lee M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J Biol Eng. 2015;9(1):1
Yin F, Cai J, Zen W, Wei Y, Zhou W, Yuan F. et al. Cartilage regeneration of adipose-derived stem cells in the TGF-β1-immobilized PLGA-gelatin scaffold. Stem Cell Rev Rep. 2015;11(3):453–9.
Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee SH, Cho H. et al. Effects of the controlled-released TGF-β1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials. 2004;25(18):4163–73.
DeFail AJ, Chu CR, Izzo N, Marra KG. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27(8):1579–85.
Saraf A, Mikos AG. Gene delivery strategies for cartilage tissue engineering. Adv Drug Deliv Rev. 2006;58(4):592–603.
Milan PB, Lotfibakhshaiesh N, Joghataie MT, Ai J, Pazouki A, Kaplan DL. et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234–46.
Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW. et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109(47):19172–7.
Kargozar S, Mozafari M, Hashemian SJ, Brouki Milan P, Hamzehlou S, Soleimani M. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: a comparative study between human mesenchymal stem cells derived from bone, umbilical cord Whartonas jelly, and adipose tissue. J Biomed Mater Res B Appl Biomater. 2016;106(1):61–72.
Wilson A, Butler P, Seifalian A. Adipose‐derived stem cells for clinical applications: a review. Cell Prolif. 2011;44(1):86–98.
Lanza R, Langer R, Vacanti J. Principles of tissue engineering. 2011. 4th Edn, Academic press: Massachusetts, USA, 1936 pp.
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54.
Baino F, Hamzehlou S, Kargozar S. Bioactive glasses: where are we and where are we going?. J Funct Biomater. 2018;9(1):25.
Knappskog S, Ravneberg H, Gjerdrum C, Trösse C, Stern B, Pryme IF. The level of synthesis and secretion of Gaussia princeps luciferase in transfected CHO cells is heavily dependent on the choice of signal peptide. J Biotechnol. 2007;128(4):705–15.
Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83(2):287–92.
Babavalian H, Latifi AM, Shokrgozar MA, Bonakdar S, Mohammadi S, Moosazadeh Moghaddam M. Analysis of healing effect of alginate sulfate hydrogel dressing containing antimicrobial peptide on wound infection caused by methicillin-resistant Staphylococcus aureus. Jundishapur J Microbiol. 2015;8(9):e28320 https://doi.org/10.5812/jjm.28320.
Mhanna R, Mhanna R1, Kashyap A, Palazzolo G, Vallmajo-Martin Q, Becher J, Möller S. et al. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng Part A. 2014;20(9-10):1454–64.
Zou Z, Sun PD. Overexpression of human transforming growth factor-β1 using a recombinant CHO cell expression system. Protein Expr Purif. 2004;37(2):265–72.
Brownh PD, Wakefield LM, Levinson AD, Sporn MB. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors. 1990;3(1):35–43.p.
Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M. Alginate sulfate—nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng. 2017;45(1):210–23.
Re’em T, Kaminer-Israeli Y, Ruvinov E, Cohen S. Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials. 2012;33(3):751–61.
Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27(1):91–99.
Cao X, Deng W, Wei Y, Yang Y, Su W, Wei Y. et al. Incorporating pTGF-beta1/calcium phosphate nanoparticles with fibronectin into 3-dimensional collagen/chitosan scaffolds: efficient, sustained gene delivery to stem cells for chondrogenic differentiation. Eur Cell Mater. 2012;23:81–93.
Guan J-L. Cell migration: developmental methods and protocols. 294. 2005. Springer Science & Business Media.
Mehlhorn AT, Niemeyer P, Kaiser S, Finkenzeller G, Stark GB, Südkamp NP, Schmal H. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12(10):2853–62.
Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. et al. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–27.
Acknowledgements
This research was generously funded by grants from the National Science Foundation (INSF) through Research Grant No. 92026749 and the Pasteur Institute of Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Askari, M., Bonakdar, S., Anbouhi, M.H. et al. Sustained release of TGF-β1 via genetically-modified cells induces the chondrogenic differentiation of mesenchymal stem cells encapsulated in alginate sulfate hydrogels. J Mater Sci: Mater Med 30, 7 (2019). https://doi.org/10.1007/s10856-018-6203-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6203-9