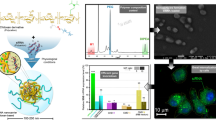
Abstract
Peptide nucleic acid (PNA) holds enormous potentials as antisense/antigenic drug due to its specific binding ability and biostability with DNA or RNA. However, the poor cellular delivery is the key obstacle in development of PNA therapy. To overcome this difficulty, we developed self-assembled nanoparticles (NPs) for delivery of PNA to living cells using amphiphilic CS derivatives. A series of N,N,N-trimethyl-O-alkyl chitosans (TMACs) with different lengths of alkyl chains were synthesized. The structures of these synthesized chemicals were characterized with FT-IR and 1H NMR. We found that the TMACs were all able to self-assemble in aqueous condition to form nano-size NPs. These nano-size NPs are spherical shape with a size range of around 100 nm and a zeta potential above +30 mV. PNA was easily encapsulated into chitosan derivative NPs by an ultrasonic method with entrapment efficiency up to 75%. The PNA-loaded TMAC NPs released the drug in a sustained manner in PBS (pH 7.4) at 37 °C. N,N,N-trimethyl-O-cetyl chitosan (TMCC) showed the best in vitro hemocompatibility and cell viability. These TMCC based NPs were able to dramatically increase the cellular uptake of PNA, specifically, 66-fold higher compared to without using these nanoparticles. The results suggest that the designed TMCC NPs might be a promising solution for improving cellular delivery of PNA.















Similar content being viewed by others
References
Luo J, Ling Y, Li X, Yuan B, Yu F, Xie WH, Chen XF. Combining amphiphilic chitosan and bioglass for mediating cellular osteogenic growth peptide gene. RSC Adv. 2015;5:79239–48.
Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–500.
Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566–8. https://doi.org/10.1038/365566a0.
Hyrup B, Nielsen PE. Peptide nucleic acids (PNA): synthesis, properties and potential applications. Bioorgan Med Chem. 1996;4:5–23.
Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry. 1995;34:11211–6.
Veldhoen S, Laufer SD, Restle T. Recent developments in peptide-based nucleic acid delivery. Int J Mol Sci. 2008;9:1276–320. https://doi.org/10.3390/Ijms9071276.
Koppelhus U, Nielsen PE. Cellular delivery of peptide nucleic acid (PNA). Adv Drug Deliv Rev. 2003;55:267–80.
Liu CD, Wang JH, Xie Y, Chen H. Synthesis and DNA/RNA complementation studies of peptide nucleic acids containing 5-halouracils. MedChemComm. 2017;8:385–9. https://doi.org/10.1039/c6md00536e.
Ellipilli S, Palvai S, Ganesh KN. Fluorinated peptide nucleic acids with fluoroacetyl side chain bearing 5-(F/CF3)-uracil: synthesis and cell uptake studies. J Org Chem. 2016;81:6364–73. https://doi.org/10.1021/acs.joc.6b01009.
Lee SH, Moroz E, Castagner B, Leroux JC. Activatable cell penetrating peptide-peptide nucleic acid conjugate via reduction of azobenzene PEG chains. J Am Chem Soc. 2014;136:12868–71. https://doi.org/10.1021/ja507547w.
Maslov MA, Kabilova TO, Petukhov IA, Morozova NG, Serebrennikova GA, Vlassov VV, Zenkova MA. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J Control Release. 2012;160:182–93. https://doi.org/10.1016/j.jconrel.2011.11.023.
Shiraishi T, Nielsen PE. Nanomolar cellular antisense activity of peptide nucleic acid (PNA) cholic acid (“Umbrella”) and cholesterol conjugates delivered by cationic lipids. Bioconjugate Chem. 2012;23:196–202. https://doi.org/10.1021/Bc200460t.
McNeer NA, Anandalingam K, Fields RJ, Caputo C, Kopic S, Gupta A, Quijano E, Polikoff L, Kong Y, Bahal R, Geibel JP, Glazer PM, Saltzman WM, Egan ME. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun. 2015;6:6952 https://doi.org/10.1038/ncomms7952.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31:603–32. https://doi.org/10.1016/j.progpolymsci.2006.06.001.
Wang H, Qian J, Ding F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J Mater Chem B. 2017;5:34.
Layek B, Haldar MK, Sharma G, Lipp L, Mallik S, Singh J. Hexanoic acid and polyethylene glycol double grafted amphiphilic chitosan for enhanced gene delivery: influence of hydrophobic and hydrophilic substitution degree. Mol Pharm. 2014;11:982–94.
Liu Y, Kong M, Cheng XJ, Wang QQ, Jiang LM, Chen XG. Self-assembled nanoparticles based on amphiphilic chitosan derivative and hyaluronic acid for gene delivery. Carbohydr Polym. 2013;94:309–16.
Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharm Sci Technol Today. 1998;1:246–52.
Prabaharan M. Review paper: chitosan derivatives as promising materials for controlled drug delivery. J Biomater Appl. 2008;23:5–36. https://doi.org/10.1177/0885328208091562.
Casettari L, Vllasaliu D, Castagnino E, Stolnik S, Howdle S, Illum L. PEGylated chitosan derivatives: synthesis, characterizations and pharmaceutical applications. Prog Polym Sci. 2012;37:659–85. https://doi.org/10.1016/j.progpolymsci.2011.10.001.
Rahmani S, Mohammadi Z, Amini M, Isaei E, Taheritarigh S, Tehrani NR, Tehrani MR. Methylated 4-N,N dimethyl aminobenzyl N,O carboxymethyl chitosan as a new chitosan derivative: synthesis, characterization, cytotoxicity and antibacterial activity. Carbohydr Polym. 2016;149:131–9.
Denora N, Lopedota A, Perrone M, Laquintana V, Iacobazzi RM, Milella A, Fanizza E, Depalo N, Cutrignelli A, Lopalco A, Franco M. Spray-dried mucoadhesives for intravesical drug delivery using N-acetylcysteine- and glutathione-glycol chitosan conjugates. Acta Biomater. 2016;43:170–84.
Nie X, Zhang J, Xu Q, Liu X, Li Y, Wu Y, Chen C. Targeting peptide iRGD-conjugated amphiphilic chitosan-co-PLA/DPPE drug delivery system for enhanced tumor therapy. J Mater Chem B. 2014;2:3232–42.
Chen JJ, Zheng LX, Chen XN, Wang ZD, Li CC, Xiao YN, Guan GH, Zhu WX. Synthesis and characterization of water-soluble chitosan grafted with hydrophilic aliphatic polyester. Int J Biol Macromol. 2015;74:433–8.
Li GB, Song P, Wang KL, Xue Q, Sui WP, Kong XZ. An amphiphilic chitosan derivative modified by deoxycholic acid: preparation, physicochemical characterization, and application. J Mater Sci. 2015;50:2634–42.
Pedro RD, Schmitt CC, Neumann MG. Syntheses and characterization of amphiphilic quaternary ammonium chitosan derivatives. Carbohydr Polym. 2016;147:97–103.
Aranaz I, Harris R, Heras A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr Org Chem. 2010;14:308–30.
Wang J, Wang L, Yu H, Zain Ul A, Chen Y, Chen Q, Zhou W, Zhang H, Chen X. Recent progress on synthesis, property and application of modified chitosan: an overview. Int J Biol Macromol. 2016;88:333–44. https://doi.org/10.1016/j.ijbiomac.2016.04.002.
Wu MM, Dong HW, Guo K, Zeng R, Tu M, Zhao JH. Self-assemblied nanocomplexes based on biomimetic amphiphilic chitosan derivatives for protein delivery. Carbohydr Polym. 2015;121:115–21.
Li DD, Pan JF, Ji QX, Yu XB, Liu LS, Li H, Jiao XJ, Wang L. Characterization and cytocompatibility of thermosensitive hydrogel embedded with chitosan nanoparticles for delivery of bone morphogenetic protein-2 plasmid DNA. J Mater Sci Mater Med. 2016;27:134 https://doi.org/10.1007/s10856-016-5743-0.
Shi YF, Xiong ZP, Lu XF, Yan X, Cai X, Xue W. Novel carboxymethyl chitosan-graphene oxide hybrid particles for drug delivery. J Mater Sci Mater Med. 2016;27:169 https://doi.org/10.1007/s10856-016-5774-6.
Kurita K, Ikeda H, Yoshida Y, Shimojoh M, Harata M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: facile preparation of a precursor useful for chemical modifications. Biomacromolecules. 2002;3:1–4.
Kean T, Roth S, Thanou M. Trimethylated chitosans as non-viral gene delivery vectors: cytotoxicity and transfection efficiency. J Control Release. 2005;103:643–53. https://doi.org/10.1016/j.jconrel.2004.01.001.
Verheul RJ, Amidi M, van der Wal S, van Riet E, Jiskoot W, Hennink WE. Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials. 2008;29:3642–9. https://doi.org/10.1016/j.biomaterials.2008.05.026.
Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173–6. https://doi.org/10.1002/jps.20627.
Huo M, Zhang Y, Zhou J, Zou A, Yu D, Wu Y, Li J, Li H. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int J Pharm. 2010;394:162–73. https://doi.org/10.1016/j.ijpharm.2010.05.001.
Yasuhara K, Kuroda K. Kinetic study of all-or-none hemolysis induced by cationic amphiphilic polymethacrylates with antimicrobial activity. Chin Chem Lett. 2015;26:479–84. https://doi.org/10.1016/j.cclet.2015.01.029.
Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability andhemolysis. Biomaterials. 2003;24:1121–31.
Douglas KL, Piccirillo CA, Tabrizian M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur J Pharm Biopharm. 2008;68:676–87. https://doi.org/10.1016/j.ejpb.2007.09.002.
Mansouri S, Cuie Y, Winnik F, Shi Q, Lavigne P, Benderdour M, Beaumont E, Fernandes JC. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27:2060–5. https://doi.org/10.1016/j.biomaterials.2005.09.020.
Liu Z, Zhang Z, Zhou C, Jiao Y. Hydrophobic modifications of cationic polymers for gene delivery. Prog Polym Sci. 2010;35:1144–62.
Acknowledgements
This work was financially supported by the Foundation and Advanced Research Project of CQ CSTC (2013jjB0011), the Innovation Project of Social Undertakings and Livelihood Security Technology of CQ CSTC (cstc2017shms-xdny0033) and Animal Disease Prevention and Food Safety Key Laboratory of Sichuan Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, C., Wang, J., Huang, S. et al. Self-assembled nanoparticles for cellular delivery of peptide nucleic acid using amphiphilic N,N,N-trimethyl-O-alkyl chitosan derivatives. J Mater Sci: Mater Med 29, 114 (2018). https://doi.org/10.1007/s10856-018-6120-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6120-y