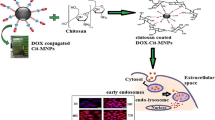
Abstract
A multifunctional drug carrier with dual targeting (magnetic and folate-receptor) and pH sensitive core-shell hybrid nanomaterial has been developed to carry an anticancer drug doxorubicin.Superparamagnetic iron oxide nanoparticles (IONPs) were used as core of the carrier and cross-linked folate conjugated chitosan (FA-CS) was acted as shell in which doxorubicin was physically entrapped. Transmission electron microscopy (TEM) analysis confirmed the average particle size of IONPs and FA-CS coated IONPs 8.2 and 15.4 nm respectively. Magnetic measurement indicated that both the IONPs and FA-CS coated IONPs were superparamagnetic at room temperature with a magnetization value 57.72 and 37.44 emu/g respectively. At pH 5.8 (malignant tissue) showed a burst release of 30.05% of the doxorubicin in the first 4 h followed by a sustained release of 88.26% of drug over 72 h. From these results it is expected that doxorubicin loaded nanoparticles can be a promising drug carrier for the treatment of solid tumors with the ability to reduce toxic side effects of drugs by selective targeting and sustained release.
Graphical abstract










Similar content being viewed by others
References
Kumar DG, Naresh R, Varma KR, Nagasen D, Sudheer B, Kishore VS. Modifications of chitosan for use in nanoparticulate drug delivery systems. Int J Pharm Sci Nanotechnol. 2013;6:2210.
Hu X, Liu S, Huang Y, Chen X, Jing X. Biodegradable block copolymer-doxorubicin conjugates via different linkages: preparation, characterization, and in vitro evaluation. Biomacromolecules. 2010;11:2094.
Rao N V, Mane S, Kishore A, Das Sarma J, Shunmugam R. Norbornene derived doxorubicin copolymers as drug carriers with pH responsive hydrazone linker. Biomacromolecules. 2011;13:221.
Vllasaliu D, Casettari L, Bonacucina G, Cespi M, Filippo Palmieri G, Illum L. Folic acid conjugated chitosan nanoparticles for tumor targeting of therapeutic and imaging agents. Pharm Nanotechnol. 2013;1:184.
Ma H, Liu Y, Shi M, et al. Theranostic, pH-responsive, doxorubicin-loaded nanoparticles inducing active targeting and apoptosis for advanced gastric cancer. Biomacromolecules. 2015;16:4022.
Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252.
Greco F, Vicent MJ, Gee S, et al. Investigating the mechanism of enhanced cytotoxicity of HPMA copolymer-Dox-AGM in breast cancer cells. J Control Release. 2007;117:28.
Yu X, Pishko MV. Nanoparticle-based biocompatible and targeted drug delivery: characterization and in vitro studies. Biomacromolecules. 2011;12:3205.
Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36:981.
Wang S, Low PS. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J Control Release. 1998;53:39.
Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 2004;96:273.
Ye W-l, Du J-b, Na R, et al. Cellular uptake and antitumor activity of DOX-hyd-PEG-FA nanoparticles. PLoS One. 2014;9:e97358.
Pillai CKS, Paul W, Sharma CP. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog Polym Sci. 2009;34:641.
Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373.
Mascolo MC, Pei Y, Ring TA. Room temperature co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials. 2013;6:5549.
Rashid TU, Rahman MM, Kabir S, Shamsuddin SM, Khan MA. A new approach for the preparation of chitosan from y irradiation of prawn shell: effects of radiation on the characteristics of chitosan. Polym Int. 2012;61:1302.
Zu Y, Zhao Q, Zhao X, Zu S, Meng L. Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method. Int J Nanomedicine. 2011;6:3429.
Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269:3198.
Li P, Wang Y, Zeng F, Chen L, Peng Z, Kong LX. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohydr Res. 2011;346:801.
Kuo C-H, Liu Y-C, Chang C-MJ, Chen J-H, Chang C, Shieh C-J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohyd Polym. 2012;87:2538.
Unsoy G, Khodadust R, Yalcin S, Mutlu P, Gunduz U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur J Pharm Sci. 2014;62:243.
Mashhadizadeh MH, Amoli-Diva M. Drug-carrying amino silane coate d magnetic nanoparticles as potential vehicles for delivery of antibiotics. J Nanomed Nanotechnol. 2012;3:2.
Jadhav N, Chodankar V. Design of chitosan based and rographolides microparticles for targetted delivery to lung tumour. Int J Pharm Pharm Sci. 2012;4:163.
Czechowska-Biskup R, Jarosinska D, Rokita Be, Ulanski P, Rosiak JM. Determination of degree of deacetylation of chitosan-comparision of methods. Prog Chem Appl Chitin Deriv. 2012;17:5.
Ahn Y, Choi EJ, Kim EH. Superparamagnetic relaxation in cobalt ferrite nanoparticles synthesized from hydroxide carbonate precursors. Rev Adv Mater Sci. 2003;5:477.
Yu BY, Kwak S-Y. Assembly of magnetite nanocrystals into spherical mesoporous aggregates with a 3-D wormhole-like pore structure. J Mater Chem. 2010;20:8320.
Vaidyanathan G, Sendhilnathan S, Arulmurugan R. Structural and magnetic properties of Co1- xZnxFe2O4 nanoparticles by co-precipitation method. J Magn Magn Mater. 2007;313:293.
Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater. 1999;201:413.
Pan C, Hu B, Li W, Sun YI, Ye H, Zeng X. Novel and efficient method for immobilization and stabilization of B-d-galactosidase by covalent attachment onto magnetic Fe3O4-chitosan nanoparticles. J Mol Catal B. 2009;61:208.
Tripathi S, Mehrotra GK, Dutta PK. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int J Biol Macromol. 2009;45:372.
Ye X-R, Daraio C, Wang C, Talbot JB, Jin S. Room temperature solvent-free synthesis of monodisperse magnetite nanocrystals. J Nanosci Nanotechnol. 2006;6:852.
Safari J, Javadian L. Chitosan decorated Fe3O4 nanoparticles as a magnetic catalyst in the synthesis of phenytoin derivatives. RSC Adv. 2014;4:48973.
Pradhan P, Giri J, Samanta G, et al. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J Biomed Mater Res Part B. 2007;81:12.
Aydin R, Pulat M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: evaluation of controlled release kinetics. J Nanomater. 2012;2012:42.
Berger JRM, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19.
Acknowledgements
The author highly acknowledge to the Ministry of Science and Technology (S&T), Bangladesh for their research grant (EAS-05) for the year 2014-2016 in the form of Special Allocation to carry out this research project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Islam, M.S., Haque, P., Rashid, T.U. et al. Core–shell drug carrier from folate conjugated chitosan obtained from prawn shell for targeted doxorubicin delivery. J Mater Sci: Mater Med 28, 55 (2017). https://doi.org/10.1007/s10856-017-5859-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5859-x