Abstract
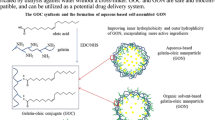
Gelatin nanoparticles, cross-linked by a mixture of a water soluble carbodiimide (CDI) and N-hydroxysuccinimide (NHS) as a non-toxic cross-linking system, was prepared. The conventional two step desolvation method with acetone as the non-solvent was used. The mean size and size distribution as well as the morphology of the formed nanoparticles were evaluated and compared with those of nanoparticles cross-linked by glutaraldehyde (GA) as the most commonly used cross-linking agent. Furthermore, intrinsic viscosities of the nanoparticles cross-linked by CDI/NHS and GA were measured and compared under various conditions. The results showed the formation of smoother and more homogeneous nanoparticles with smaller size when CDI/NHS used as cross-linking agent under the same synthesis condition. Moreover, nanoparticles encapsulating paracetamol as a model drug were produced by the two different cross-linking agents and were characterized for drug entrapment and loading efficiencies and in vitro drug release. Both drug entrapment and loading efficiencies was higher in the CDI/NHS cross-linked nanoparticles; however, the release kinetics was comparable to that of nanoparticles cross-linked with GA. The differences in the characteristics of CDI/NHS and GA cross-linked nanoparticles were attributed to the different nature of network structures formed by the two cross-linking agents. On the whole, these results suggested that CDI/NHS cross-linked nanoparticles have high potential to be used for drug delivery application in preference to the nanoparticles synthesized by toxic cross-linking agents.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.




Similar content being viewed by others
References
Schrieber R, Gareis H. Gelatine handbook. Weinheim: Wiley-VCH; 2007.
Schwick HG, Heide K. Immunochemistry and immunology of collagen and gelatin. Bibl Haematol. 1969;33:111–25.
Zwiorek K, Kloeckner J, Wagner E, Coester C. Gelatin nanoparticles as a new and simple gene delivery system. J Pharm Pharmaceut Sci. 2004;7:22–8.
Balthasar S, Michaelis K, Dinauer N, von Briesen H, Kreuter J, Langer K. Preparation and characterisation of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials. 2005;26:2723–32.
Coester CJ, Langer K, von Brisen H, Kreuter J. Gelatin nanoparticles by two-step desolvation—a new preparation method, surface modifications and cell uptake. J Microencapsul. 2000;17:187–93.
Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm. 2004;57:251–61.
Saxena A, Sachina K, Bohidar HB, Kamra Verma A. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf B: Biointerfaces. 2005;45:42–8.
Azarmi Sh, Huang Y, Chen H, McQuarrie S, Abrams D, Roa W, Finlay WH, Miller GG, Löbenberg R. Optimization of a two-step desolvation method for preparing gelatin nanoparticles and cell uptake studies in 143B osteosarcoma cancer cells. J Pharm Pharmaceut Sci. 2006;9:124–32.
Jahanshahi M, Sanati MH, Hajizadeh S, Babaei Z. Gelatin nanoparticle fabrication and optimization of the particle size. Phys Status Solidi (a). 2008;205:2898–902.
Won YW, Kim YH. Preparation and cytotoxicity comparison of type A gelatin nanoparticles with recombinant human gelatin nanoparticles. Macromol Res. 2009;17:464–8.
Saraogi GK, Gupta P, Gupta UD, Jain NK, Agrawal GP. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int J Pharmaceut. 2010;385:143–9.
Ofokansi K, Winter G, Fricker G, Coester C. Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur J Pharmaceut Biopharmaceut. 2010;76:1–9.
Oppenheim RC, Stewart NF. The manufacture and tumour cell uptake of nanoparticles labelled with fluorescein isothiocyanate. Drug Dev Ind Pharm. 1979;5:563–71.
Leo E, Vandelli MA, Cameroni R, Forni F. Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: involved of the drug in the cross-linking process. Int J Pharmaceut. 1997;155:75–82.
Leong KW, Mao HQ, Truong VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release. 1998;53:183–93.
Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J Mater Sci Mater Med. 2002;13:523–6.
Bajpai AK, Choubey J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J Mater Sci Mater Med. 2006;17:345–58.
Ethirajan A, Schoeller K, Musyanovych A, Ziener U, Landfester K. Synthesis and optimization of gelatin nanoparticles using the miniemulsion process. Biomacromolecules. 2008;9:2383–9.
Kulicke WM, Clasen C. Viscosimetry of polymers and polyelectrolytes. Berlin: Springer; 2004.
Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharmaceut Res. 2009;26:1025–58.
Mohanty B, Bohidar HB. Systematic of alcohol-induced simple coacervation in aqueous gelatin solutions. Biomacromolecules. 2003;4:1080–6.
Olde Damink LHH, Dijkstra PJ, van Luyn MJA, van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as crosslinking agent for collagen based biomaterials. J Mater Sci Mater Med. 1995;6:460–72.
Farris S, Song J, Huang Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J Agric Food Chem. 2010;58:998–1003.
Khor E. Methods for treatment of collagenous tissue for bio-prostheses. Biomaterials. 1997;18:95–105.
Kuijpers AJ, Engbers GHM, Feijen J, De Smedt SC, Meyvis TKL, Demeester J, Krijgsveld J, Zaat SAJ, Dankert J. Characterization of the network structure of carbodiimide cross-linked gelatin gels. Macromolecules. 1999;32:3325–33.
Timkovicz R. Detection of the stable addition of carbodiimide to proteins. Anal Biochem. 1997;79:135–43.
Yamakawa H. Concentration dependence of polymer chain configurations in solution. J Chem Phys. 1961;34:1360–72.
Gupta A, Mohanty B, Bohidar HB. Flory temperature and upper critical solution temperature of gelatin solutions. Biomacromolecules. 2005;6:1623–7.
Acknowledgment
Partial financial support from the Iranian Nanotechnology Initiative and vice-president for research and technology of University of Tehran is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Professor Mohammad-Nabi Sarbolouki (1949–2009).
Rights and permissions
About this article
Cite this article
Taheri Qazvini, N., Zinatloo, S. Synthesis and characterization of gelatin nanoparticles using CDI/NHS as a non-toxic cross-linking system. J Mater Sci: Mater Med 22, 63–69 (2011). https://doi.org/10.1007/s10856-010-4178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4178-2